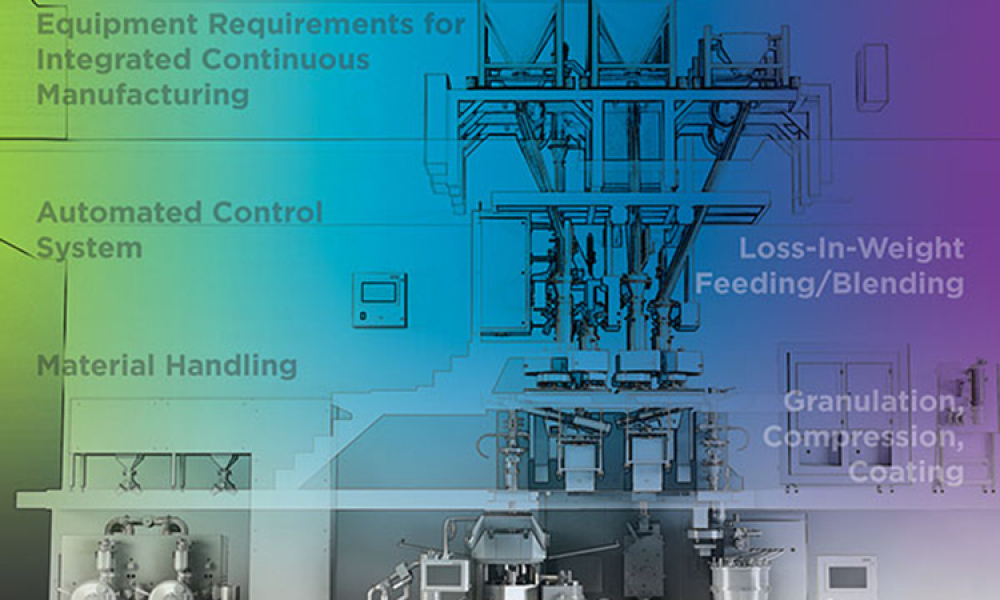
As the benefits of continuous manufacturing became more apparent in the pharmaceutical industry, companies looked for ways to incorporate the technology into their manufacturing processes. In 2017 The ISPE Oral Solid Dosage (OSD) Community of Practice (CoP) formed a working team to advance the use of continuous manufacturing in the pharmaceutical industry and to increase the long-term...
Guidance Documents
Information about ISPE's Guidance Documents, white papers, Knowledge Briefs and other written technical guidance.
iSpeak Blog
iSpeak Blog
For nearly a century, production of Water for Injection (WFI) was universally accepted to be distillation-based. As emphasis on costs and environmental concerns has grown, pharmacopeias around the world have focused on the quality attributes of WFI to allow for consideration of other production technologies. In 2017, the European Pharmacopoeia joined the US, Japan, and many other regulatory...
iSpeak Blog
What is the current state of your organization’s Change Management (CM) System? The effective management of change throughout the product lifecycle enables quality improvement and is critical to patient safety, supply reliability, as well as operational effectiveness and efficiency.