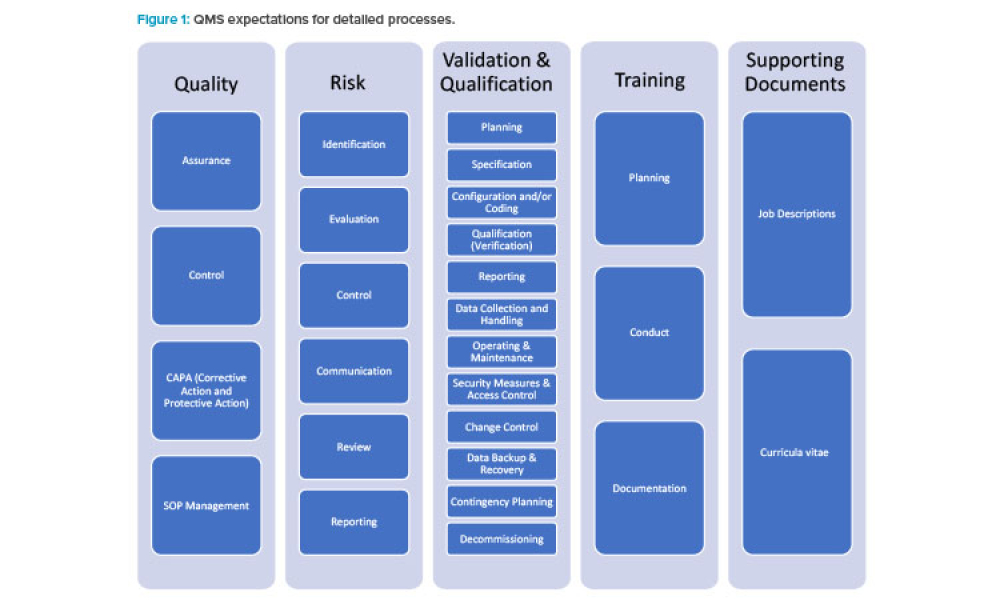
Existing risk-based approaches to computerized system compliance and validation as outlined in GAMP® 5
September / October 2020
Because of the global pandemic, we have experienced unexpected joys, learned new skills, adjusted to long days of video conferencing, and dealt with drops in income and potential job losses. At the same time, we have also experienced an increased sense of urgency, collaboration, and pride because we are a part of the industry that has been tapped to heal all our nations from this unexpected...
As many of you read this, I am sure your social and work life have been turned upside down, flipped around, and now are possibly settling into “the new normal.” I personally cringed when I wrote that—I was so tired of hearing that phrase about three weeks into the pandemic.
In the pharmaceutical industry, digitalization involves developing and implementing digital technologies at all levels of pharmaceutical operations. The aim is to transform the industry by capturing, analyzing, and using vast amounts of data collected from a wide range of sources to support research and development (R&D), clinical development, drug manufacturing, supply chain management,...
How many times have you heard phrases like these over the last several months: unprecedented marketplace disruptions, staggering economic conditions, or maybe insurmountable business challenges? If you’re like me, probably more than you can count.