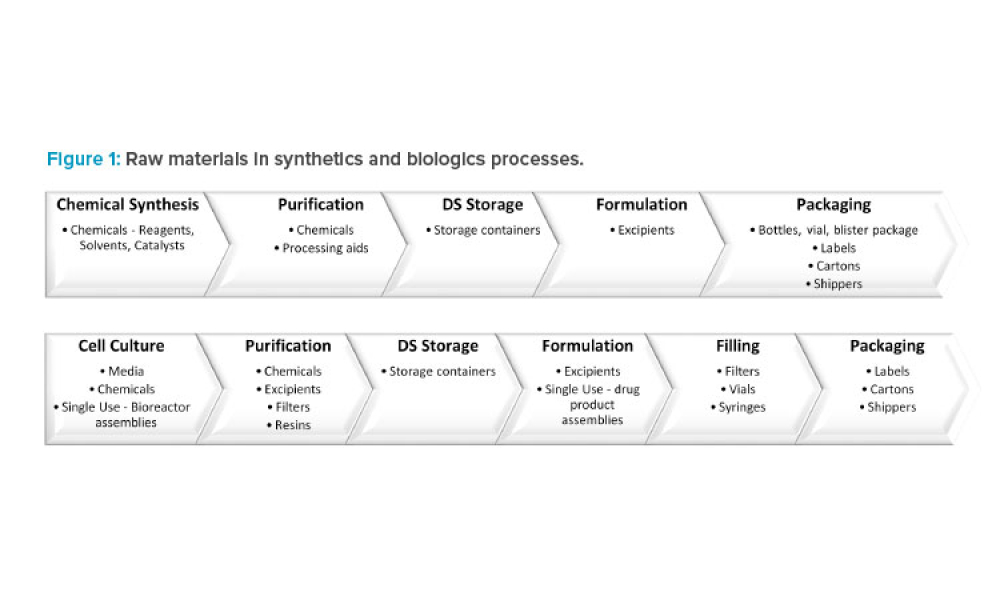
Pandemic-related supply chain shortages have placed constraints on the supply of essential filters and chromatography resins. An agile regulatory pathway to implement alternative filters and resins into manufacturing is necessary to ensure the continued supply of approved biologics. To allow this in the US and potentially globally, the regulatory strategy proposed in this article is to provide...
Tabetha Bonacci, PhD

Amgen
Director in Regulatory Affairs-CMC
Tabetha M. Bonacci, PhD, is a Director in Regulatory Affairs-CMC at Amgen, Inc. She co-leads the Lifecycle Management group overseeing regulatory strategy and submissions for Amgen’s commercial portfolio. Her areas of interest include developing novel science- and risk-based approaches for managing postapproval changes, and harmonization of regulatory expectations for postapproval change management. She also works to develop robust processes and procedures for developing and managing regulatory strategy and submissions for commercial products. Tabetha holds a BS in biology and chemistry from St. Norbert College and a PhD in pharmacology from the University of Rochester.