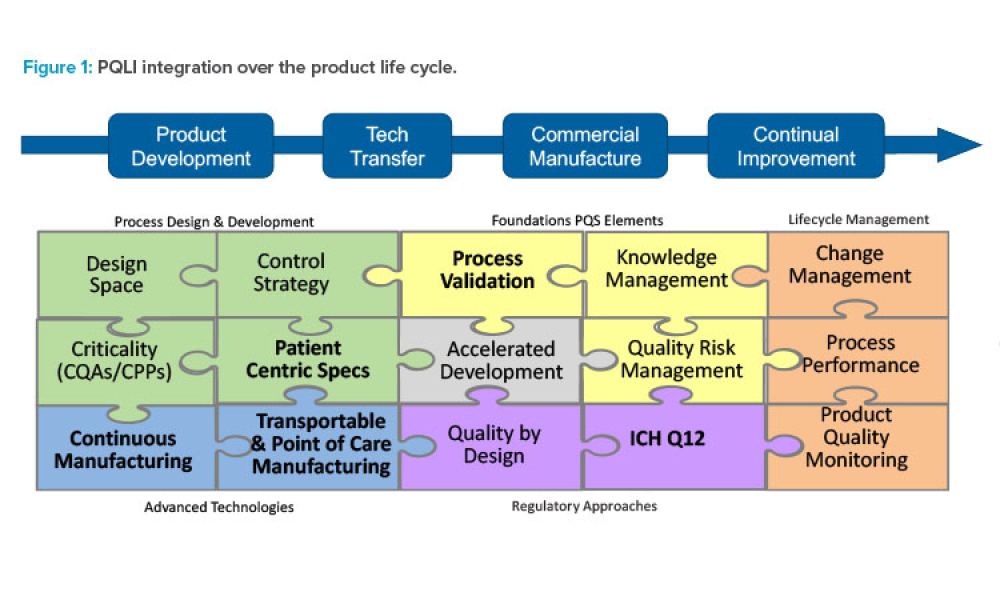
A unique aspect of the pharmaceutical industry is the pairing of innovation and regulation. For nearly two decades, ISPE’s Product Quality Lifecycle Initiative (PQLI®) has worked at the nexus of...
Eli Zavialov, PhD

GlaxoSmithKline
Senior Director
Eli Zavialov, Ph.D., is a Senior Director, CMC Regulatory Affairs at GlaxoSmithKline. In his current role, Eli leads a team of regulatory professionals managing a diverse portfolio of biological products. He is responsible for the development and implementation of effective regulatory strategies, delivery of global dossier submissions, and managing direct agency interface for CMC related aspects. Prior to this role, Eli held positions of increasing responsibility within technical and regulatory groups at Johnson & Johnson, Merck, and Schering-Plough. Eli holds a PhD in organic and bioorganic chemistry from the University of Southern California, and BSc in chemistry from Moscow State University, Moscow, Russia. He joined ISPE in 2019.