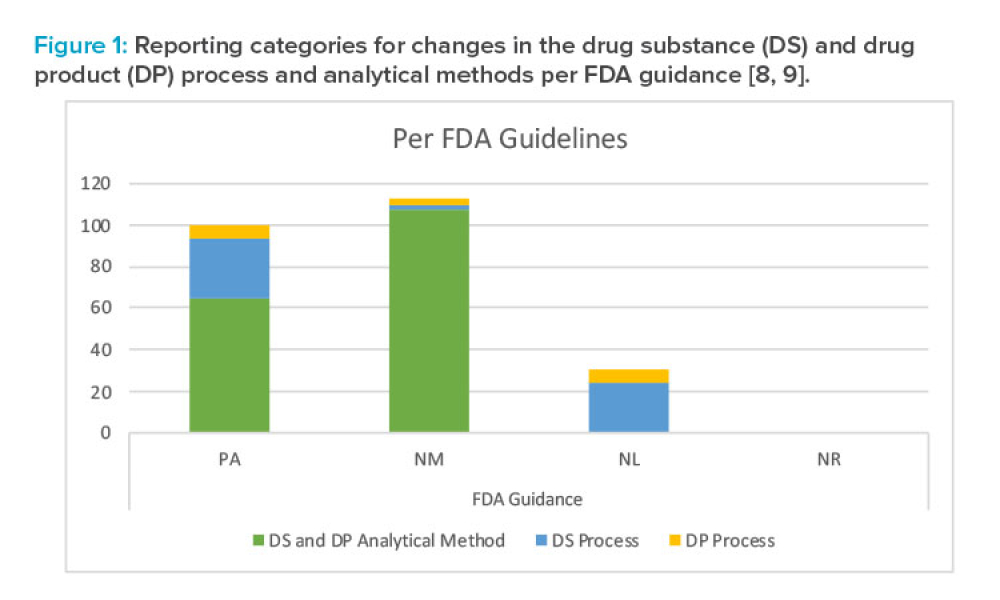
The ICH Quality Discussion Group (QDG) has recommended contemporizing the specification setting guidelines ICH Q6A and Q6B. This provides an excellent opportunity to update ICH Q6A/B to align and work in a complementary manner with the science-, risk/knowledge-based concepts in ICH Q8–Q12. The ISPE Pharmaceutical Quality Lifecycle Implementation (PQLI) Patient-Centric Specification working...
Online Exclusives