Quality Management Systems: Agile Approach for Product Realization & Lifecycle Management (T57) - New Course!
Overview
The Pharmaceutical Quality System (PQS), supported by ICH Q10, is the key foundation on which product realization depend. Through lecture and group exercises this course illustrates how quality systems work, the purpose of the different elements, how they connect to each other and how to recognize and transfer knowledge/connectivity throughout the organization. The diagram below from ICH Q10, covers the product life cycle for a PQS/QMS system and all aspects will be covered by this course. We will be using QMS and PQS terms interchangeably throughout this course to establish a holistic approach.
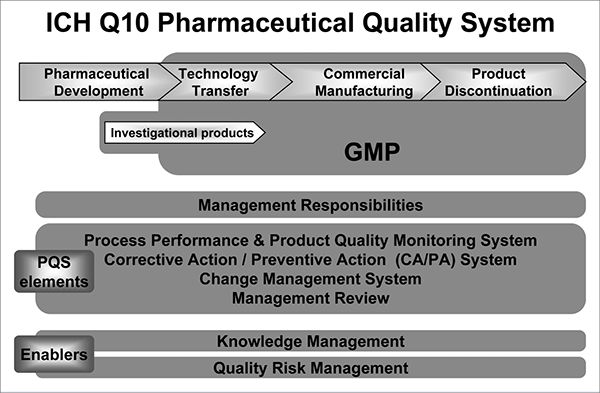
Source: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonized Tripartite Guideline, Pharmaceutical Quality System Q10
What You Will Learn
- International regulatory PQS guidelines from EMA, USFDA and PIC/s and related regulatory actions to understand how compliance, science and risk both depend on the QMS/PQS.
- Background, objectives and the business benefits of ICH documents:
- Q10 Pharmaceutical Quality System
- Q9 Quality Risk Management
- Q8 Pharmaceutical Development
- Q11 Development and Manufacture of Drug Substances
- Q12 Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (draft)
- The PQS’ role in quality and regulatory phases of the lifecycle including development, technology transfer, manufacture and discontinuation.
Resources and Activities
- Pre-Course Work Materials
- Interactive Exercises
- Learning Assessments
- ISPE PQLI® Guide Series: Part 3, Change Management System as a Key Element of a Pharmaceutical Quality System
- Part 4, Process Performance and Product Quality Monitoring System
Course Modules
- Introduction ICH Q10 background, regulatory, lifecycle & elements
- Regulatory & Q10 Background
- Breakout Scenario - Warning Letters
- Summary of Q10
- Breakout Scenario - ICH Q10
- Enablers
- Knowledge management and quality risk management
- Management Responsibilities & Outsourced activities
- Breakout Scenario: Management Responsibilities
- Product Performance & Product Quality Monitoring System (PPPQMS)
- Breakout Scenario: Development and manufacturing for API, a new product, a legacy product
- Using the process performance and pharmaceutical quality system to drive continual improvement
- CAPA and Change Management and Change Management and link to QRM
- Breakout Scenarios: Change Management
- Management Review
- Quality Culture Module
- Tools and concepts
Who Should Attend
- Quality Assurance
- Tech Support
- Tech transfer functions
- Operations functions
- Early career (3-5 years within the industry)
- Qualified persons in Europe
- Team Lead/Operator/Technician Staff
- Managers
- Section heads
- Supervisors and CEOs
- President
- Owner or General Manager working in pharmaceutical and biotech manufacturing (not CMO)
- Engineering
- Architecture or construction
- Service provider or consulting
- Equipment or material supplier
Addtional Course Details
Learning Objectives
- Apply ICH Q10 terminology, concepts, and implementation to provide meaningful application for determining the
objectives and enablers for:- Product Realization, State of Control, PPPQMS, QRM
- Q9 Quality Risk Management
- Knowledge Management
- Product Steward
- Management Responsibility: Management Review, Outsourced activities
- Continual Improvement: CAPA, Change Management
- Apply ICH Q9 philosophy, principles and risk management to assess the impact:
- Process Understanding
- CAPA
- Continual improvement
- Change Management, including
- EU: Type IA, IB, II; Post Approval Change Management Protocols and US: CBE, Post
Approval Supplements, Annual Reportable, Comparability Protocols
- EU: Type IA, IB, II; Post Approval Change Management Protocols and US: CBE, Post
- Discuss the organization and use of external parties; how to get the best from similarities and differences between PQS systems in Contract givers and Contract receivers; monitoring suppliers (e.g. technical agreements, ‘ownership’ of supply chain, differing views of Stage 3 CPV scope).
- Understand State of Control, what it means and how it relates to:
- Validation and CPV/OPV, APQR
- PV lifecycle Stage 3
- Technology Transfer
- Understand the importance of establishing a cross-functional quality culture for successful PQS implementation and to ensure product quality by applying the tools provided.


