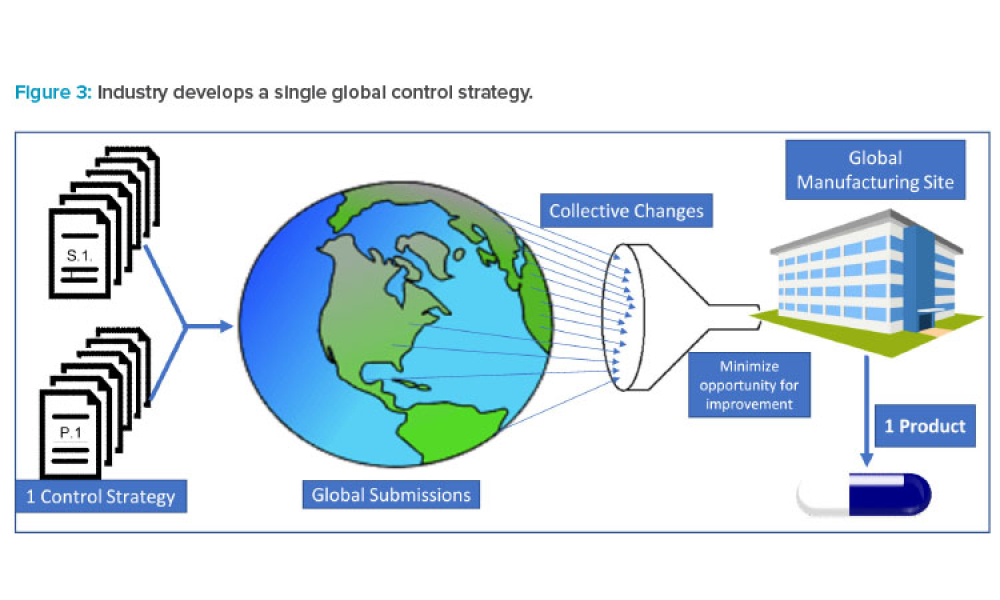
Pharmaceutical and biotechnology companies employ platform analytical procedures in the development stages of their synthetic and biological drug products and are beginning to leverage them for commercial products. This shift is supported by the acceptance of platform procedures in the recently adopted ICH Q2(R2) and ICH Q14. Six case studies are shared in this article to highlight how...
Timothy Graul

Pfizer, Inc
Director in the Global CMC Advisory Office
Timothy W. Graul is a Director in the global CMC advisory office at Pfizer, Inc. At Pfizer, Tim supported the development of drug product formulations and drug substance synthetic routes by providing analytical methods, data, and strategy as a member of analytical R&D. Tim has been recognized as a leader in Quality by Design for analytical methods and has collaborated on publications in this area. He was recently appointed as the PhRMA Deputy Topic lead on the ICH Q2/Q14 Expert Working Group. Tim has been a member of industry groups including Land O’ Lakes Pharmaceutical Analysis Conference Planning Committee, IQ groups such as Analytical Leadership, AQbD, Dissolution, Control Strategy Harmonization Metrics, and Worldwide Specification Harmonization. He holds a PhD in Analytical Chemistry from Florida State University.