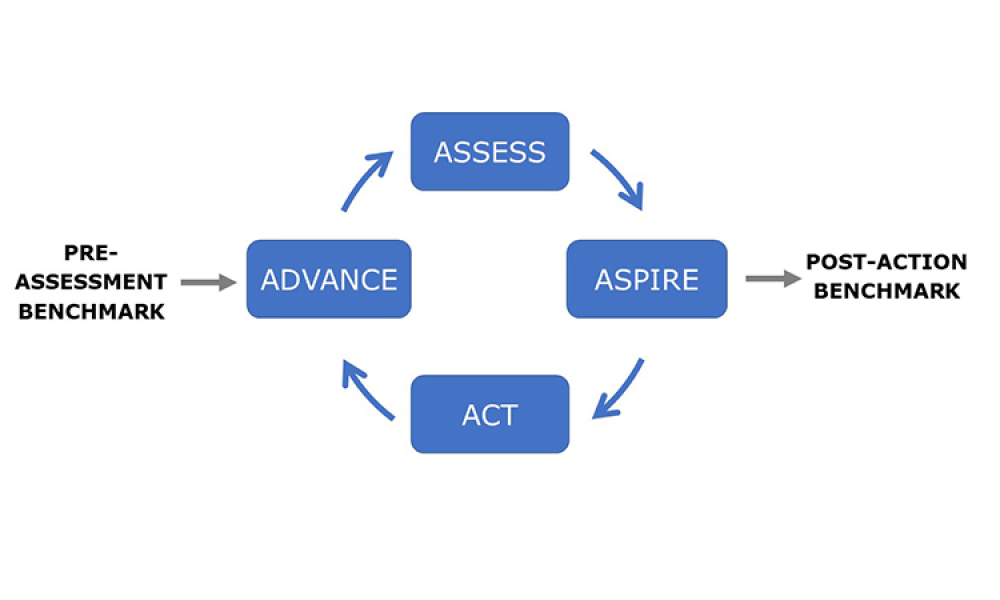
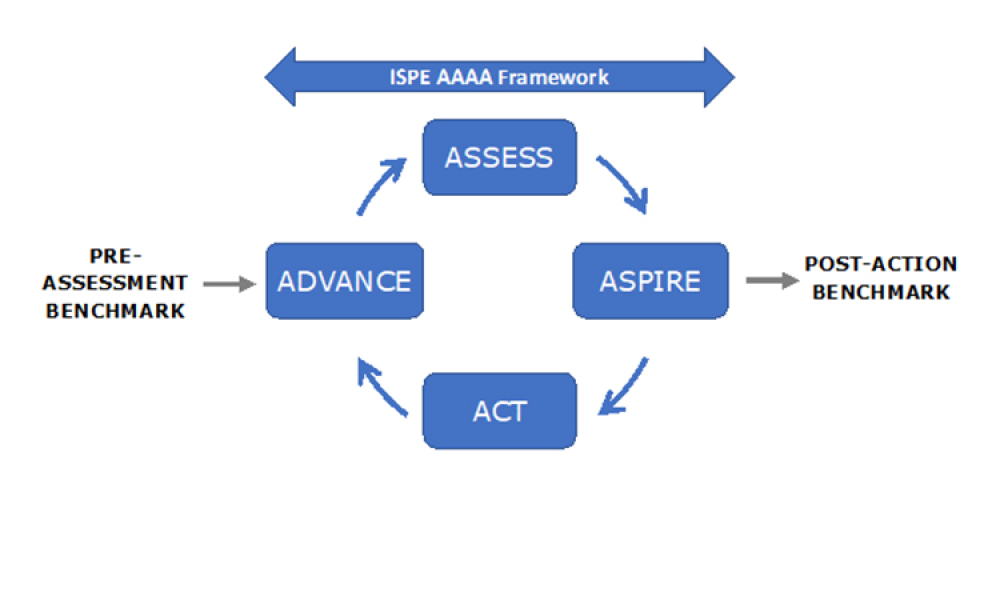
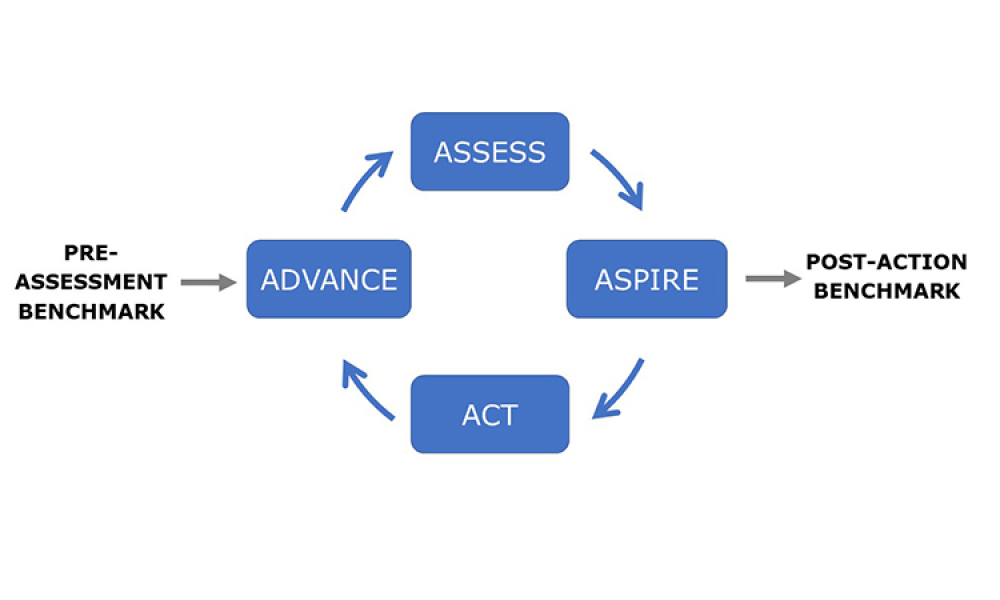
Perrigo
Senior Director, Corporate Quality
Tami is Senior Director of Corporate at Perrigo. She the Chair of ISPE's Advancing Pharmaceutical Quality team. Tami is a co-author of the 2022 ISPE APQ Series, Quality Management Maturity Program five guide series. Tami led the PDA/ISPE Culture Collaboration team authoring the Root Cause Analysis Guide in 2019 and co-authored ISPE Cultural Excellence report in 2017. She is a member of the ISPE Regulatory Steering Team.
Tami is Global Quality Leader with 30 years of experience in the Pharmaceutical Industry. She is a chemical engineer by trade and cultural excellence consultant. Tami has held positions in Corporate Quality, QA & QC Operations, Regulatory Affairs, R&D, Operational Excellence, and Technical Engineering. She is a certified Lean Six-Sigma Blackbelt and a Certified Quality Engineer. She is also HACCP certified and a Safe Quality Food Practitioner. Her experience spans solid, semi-solid, and liquid drug dosage forms, API’s, Medical Device, and Infant Formula/Foods. Tami has led multicultural Quality teams in the implementation of global quality systems such as global change control, EDMS, SQM, quality investigation and CAPA, corporate quality metrics & governance, global technology councils, and auditing to support compliance. She is a strategic, global continuous improvement leader and change agent advancing the culture of quality throughout all levels in the organization.