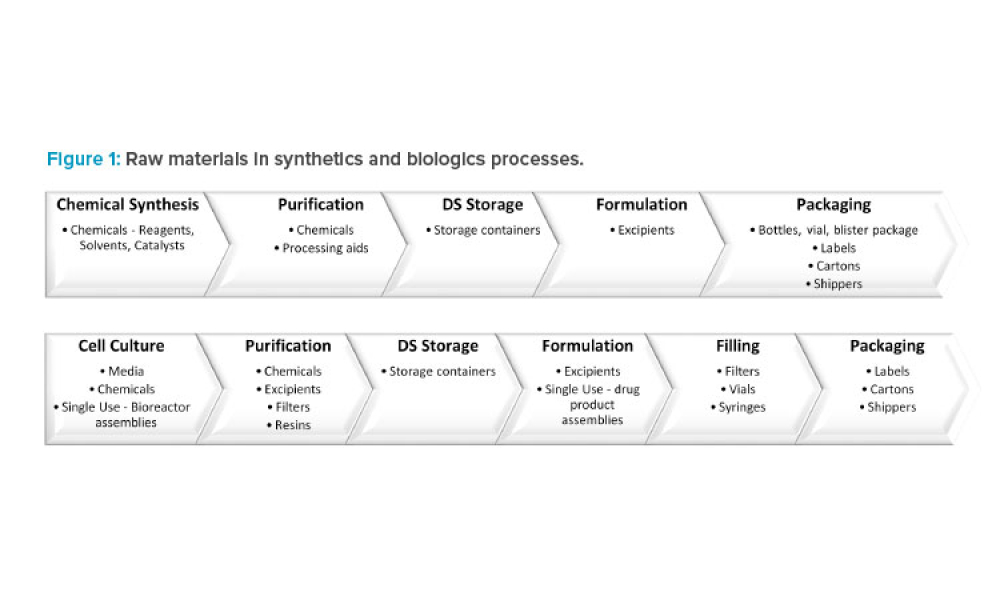
A reliable supply of raw materials is critical to maintain a robust supply chain to serve patients globally. With shortages, regulatory complexity is compounded due to differences in submission and data requirements from various regulatory agencies. Therefore, there is an increasing need to implement a harmonized regulatory infrastructure that is both flexible and predictable to provide more...
Features