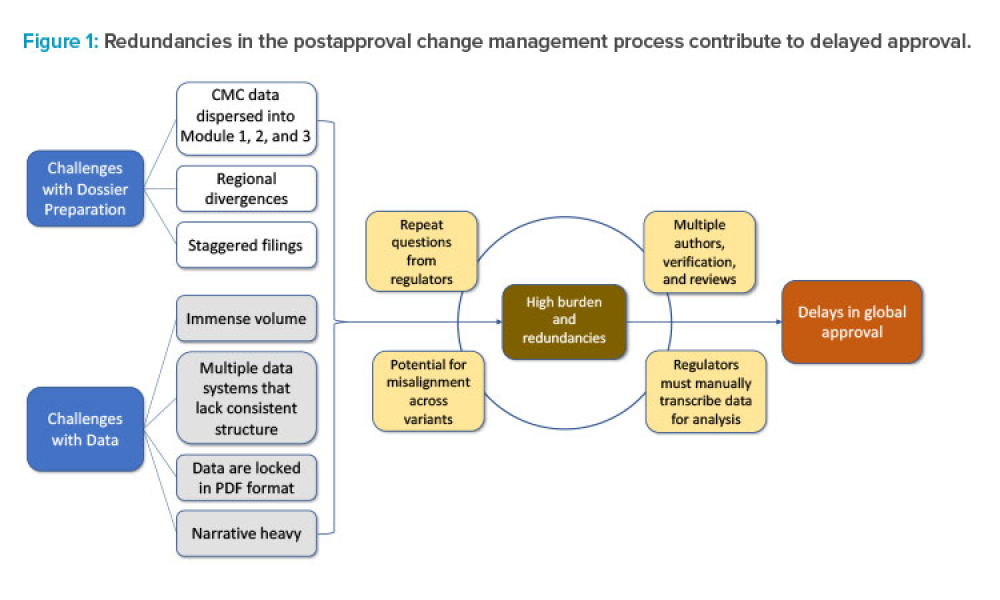
The ISPE 2023 Annual Meeting & Expo agenda is always packed with sessions offering pharmaceutical professionals opportunities to engage in industry-critical conversations. Several of ISPE's regulatory initiatives took center stage during the technical sessions in the Regulatory and Quality track at the
Jessica Lo Surdo, PhD

Amgen
Regulatory Affairs Senior Manager
Jessica Lo Surdo, PhD, is a Regulatory Affairs Senior Manager at Amgen, Inc., focusing on CMC strategies for global clinical trial applications and postapproval variations for biologics. Jess has been at Amgen for three years, leading commercial product CMC regulatory strategies, with recent focus on established conditions and ICH Q12 implementation through ISPE. She previously worked at Bristol Myers Squibb for 2.5 years as an analytical product lead scientist, leading analytical aspects of prepivotal clinical programs. Jess was at the FDA Center for Biologics Evaluation and Research in the Division of Cellular and Gene Therapies for eight years, with initial focus on bioassay development for cellular therapies, and later as a Staff Fellow and Product Quality Reviewer for cell therapy INDs. Jess holds PhD and master’s degrees in bioengineering from the University of Pittsburgh, and a BS degree in bioengineering from Syracuse University. She has been an ISPE member since 2020.