In April 2023, ISPE launched a survey to understand the sources of barriers to technological innovation within the pharmaceutical industry. This survey is part of an expansive and significant initiative by ISPE, Enabling Global Pharma Innovation: Delivering for Patients, which aims to promote consistent and harmonized interpretation and implementation of guidelines issued by the International...
Gregory Rullo

Related Articles
The pharmaceutical industry faces considerable challenges throughout the development, manufacturing, and supply of medicines, largely due to the intricate and divergent global regulatory landscape. The adoption of structured data standards and utilization of cloud-based platforms offer immense potential to overcome these challenges by facilitating faster and more efficient global...
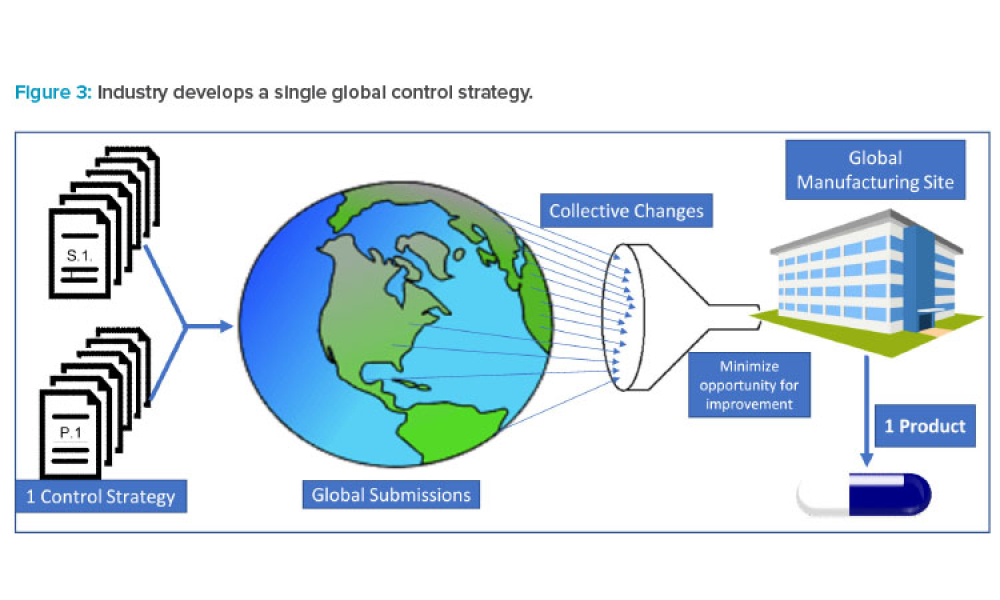
During the past decade, industry has experienced a proliferation of regulatory divergence regarding the interpretation and implementation of International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines (and control strategies) across geographic regions. This article shares data that highlight instances where well-established ICH...
A thought-provoking session held during the 2021 ISPE Annual Meeting & Expo provided insight into how the Overall Quality Summary can provide an ideal opportunity to provide a...