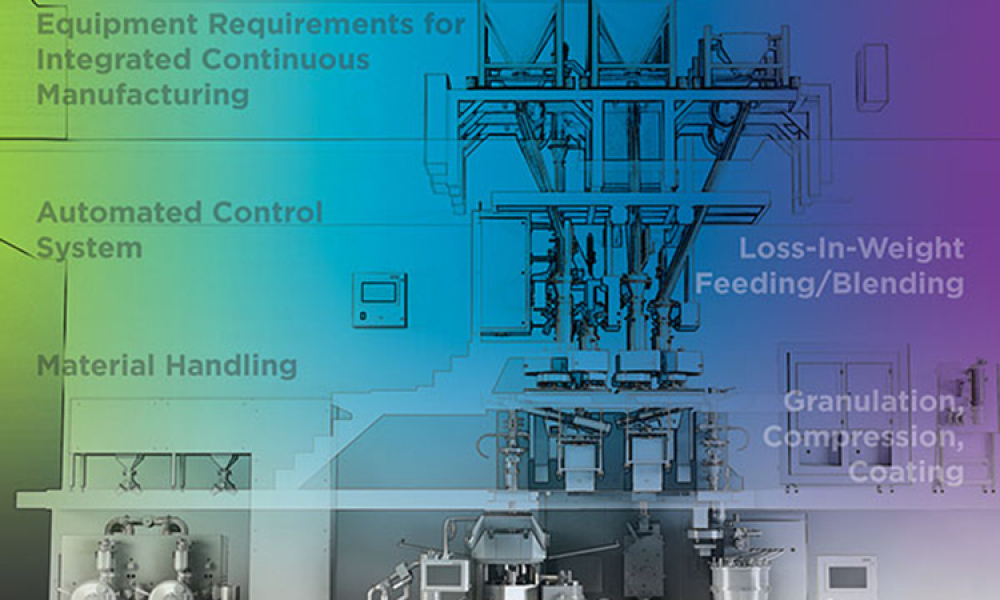
As the benefits of continuous manufacturing became more apparent in the pharmaceutical industry, companies looked for ways to incorporate the technology into their manufacturing processes. In 2017 The ISPE Oral Solid Dosage (OSD) Community of Practice (CoP) formed a working team to advance the use of continuous manufacturing in the pharmaceutical industry and to increase the long-term...
iSpeak Blog