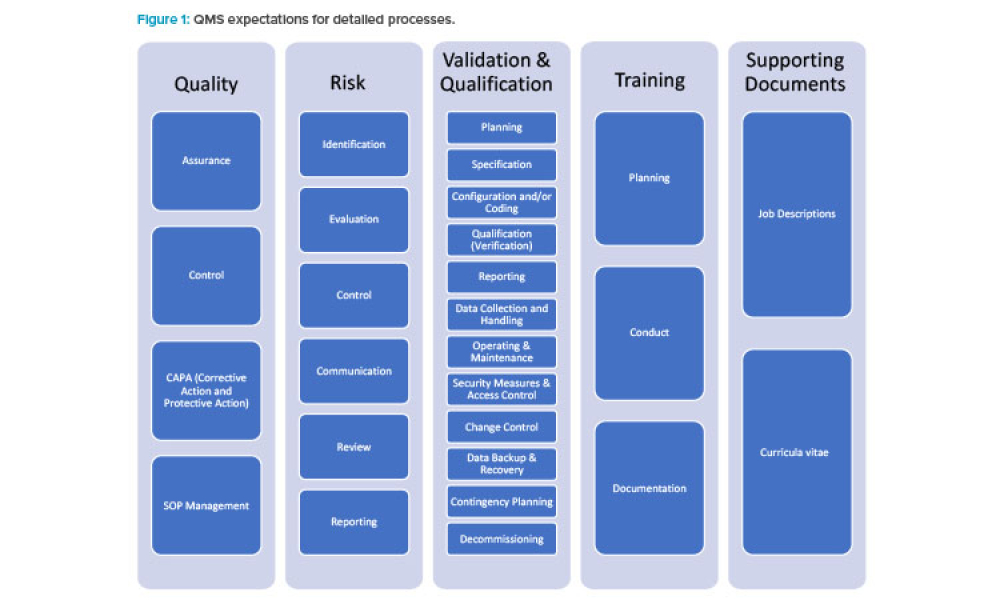
GAMP® 5 (Second Edition) was published on 29th July 2022 and was presented and discussed at the 2023 ISPE Annual Meeting and at several local...
Frank Henrichmann

Q-FINITY Quality Management
Senior Executive Consultant
Frank Henrichmann, Senior Executive Consultant at Q-FINITY Quality Management, is an expert in quality management, computer system validation, and compliance, especially in the context of clinical trials and pharmacovigilance. Over more than 22 years, he has gained extensive experience in strategies, projects, and measures for GxP-regulated environments at a CRO as well as a major pharmaceutical company. In his current position, he helps life sciences companies and supports technology providers to find innovative answers to quality and validation challenges. Frank is a member of the Clinical Systems Special Interest Group (SIG) and is a coauthor of the GAMP® Good Practice Guide: Validation and Compliance of Computerized GCP Systems and Data. He has been an ISPE member since 2001 and currently is the Co-Chair of the GAMP Global Steering Committee.