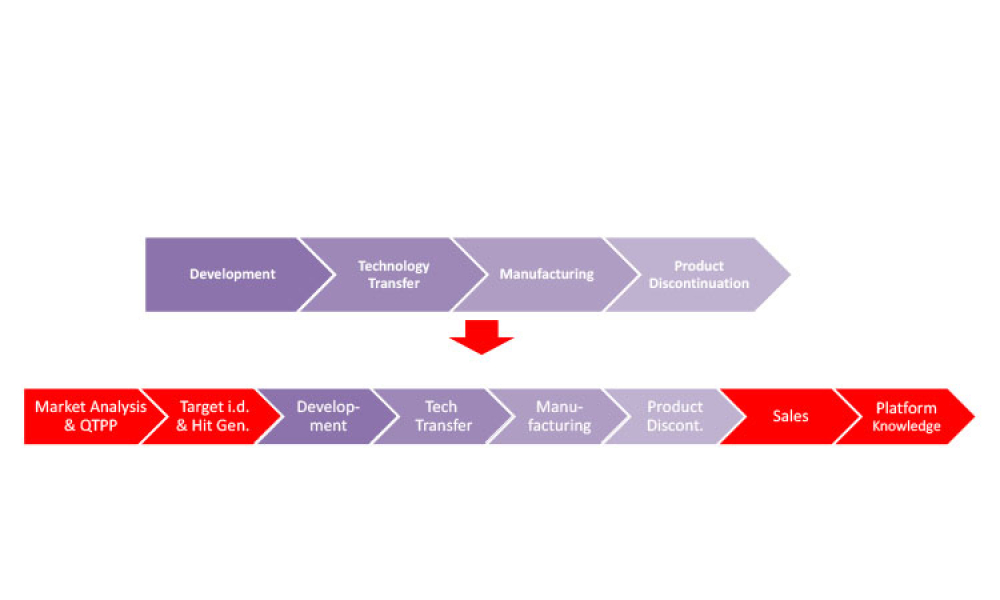
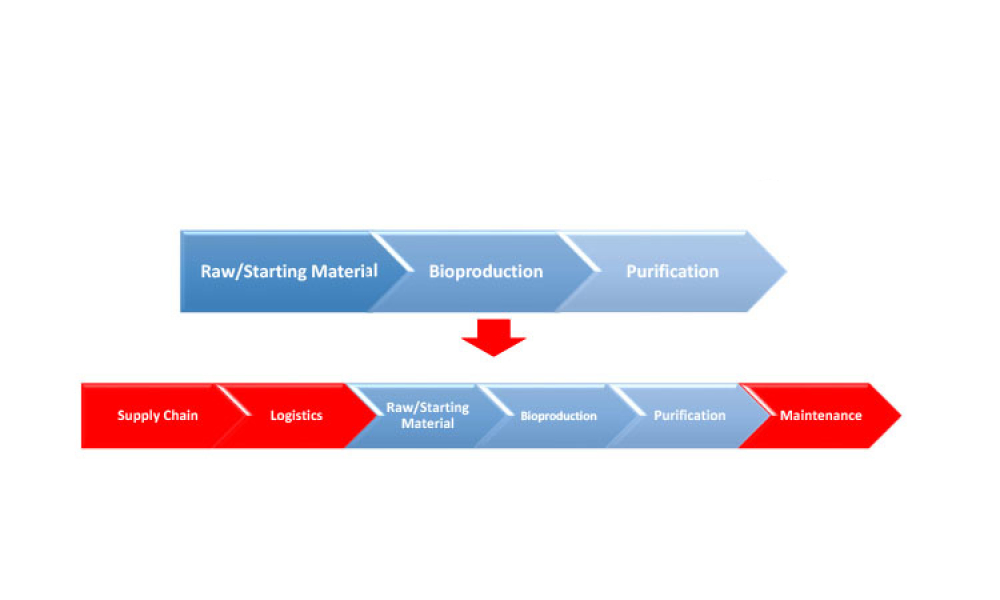
This second of a two-part series explores digital transformation and digitalization in the biopharmaceutical industry with information about how data science enables digitalization along the product life cycle. (Part 1 was published in the March-April 2021 issue of Pharmaceutical Engineering.
Features