Features
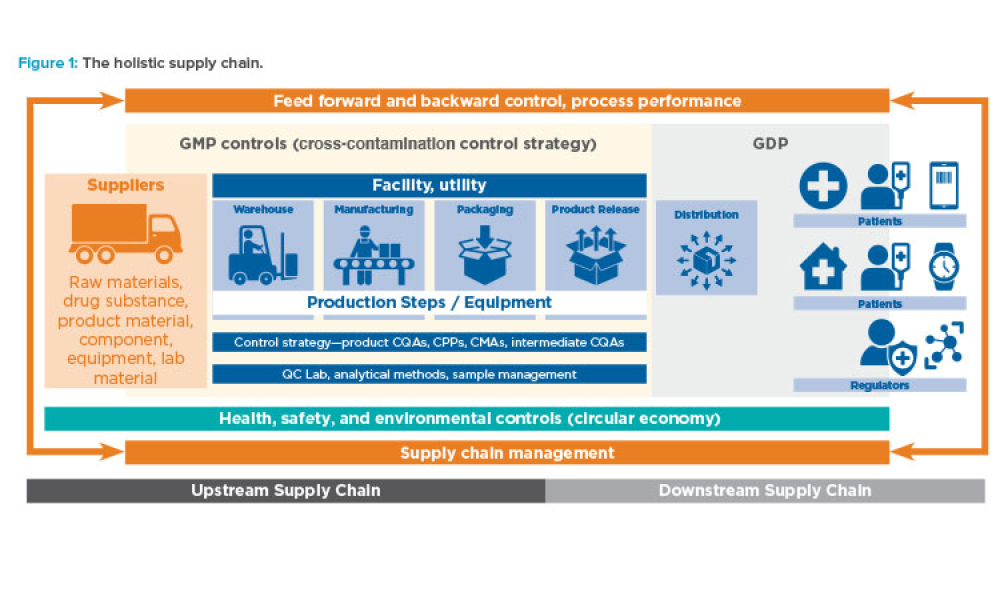
The fourth Industrial Revolution (also known as Industry 4.0) is the era of smart machines, storage systems, and production plants that can autonomously exchange information, trigger actions, and control operations free of any human intervention. To ensure future success in the delivery of therapeutic medicines to patients, it is imperative that the pharmaceutical industry move deeper into the...