In the pharmaceutical industry, ensuring the safety, quality, and efficacy of products is paramount. With the increasing complexity of manufacturing processes, the growing reliance on computerized systems and advancements in technology, GAMP® guidance provides a framework for validating and maintaining the integrity of computerized systems, ensuring they are fit for their intended...
Christopher Reid

Related Articles
Risk assessment is essential, however, risk assessment is of limited value if it is not conducted by a team with the necessary process, product, and functional understanding. Conducting risk assessments prematurely may lead to invalid assessment of overall risks. Conducting risk assessments too late will limit the opportunity to address design flaws and effectively test processes and...
This article describes a practical and pragmatic approach to the management of computerized system life cycle and information technology (IT) process records. The objective is to effectively achieve and maintain compliant GxP-regulated systems that are fit for intended use, and to support patient safety, product quality, and data integrity.
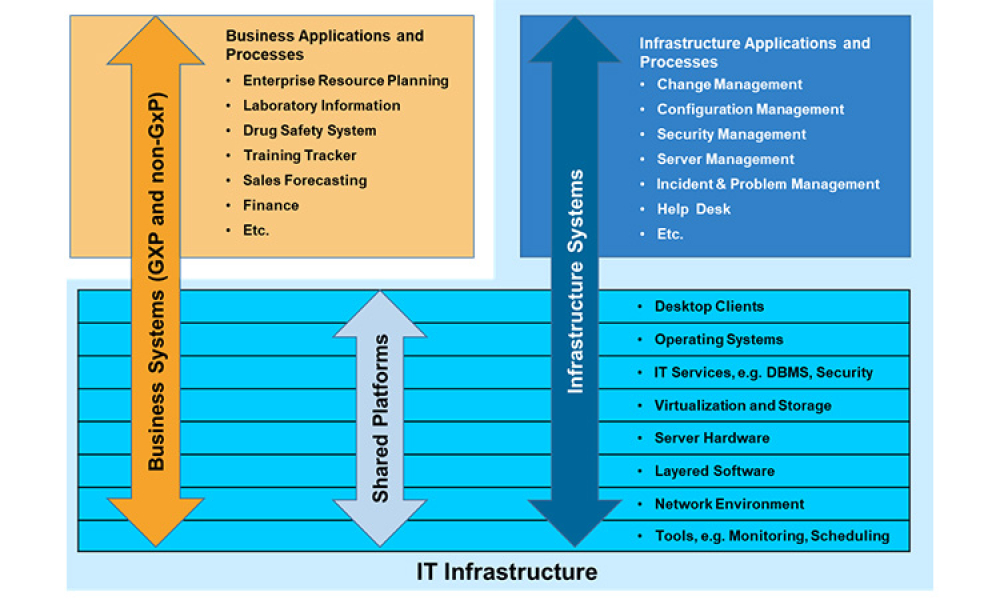
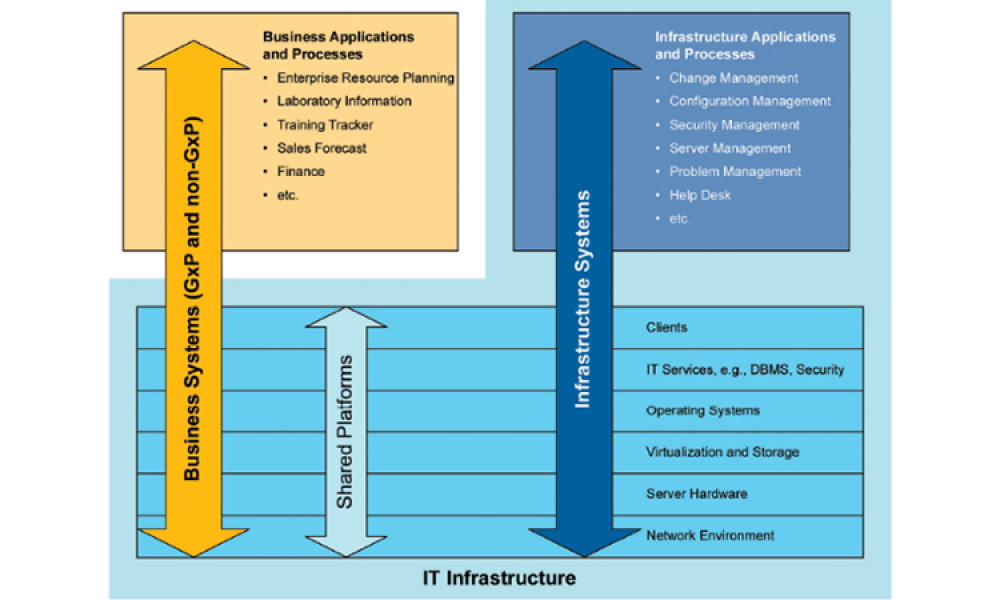
This article focuses on pragmatic quality- and risk-based approaches to IT infrastructure. It covers recommendations made by a US FDA/industry team linked to the US FDA Center for Devices and Radiological Health (CDRH) Case for Quality initiative
In the previous blog, GAMP® Support for Pragramtic Quality & Risk-Based Approaches, we saw how as part of the Case for Quality program US FDA CDRH (Center for Devices and...