CAPA / RCA / Investigations Training Course (T73)
Overview
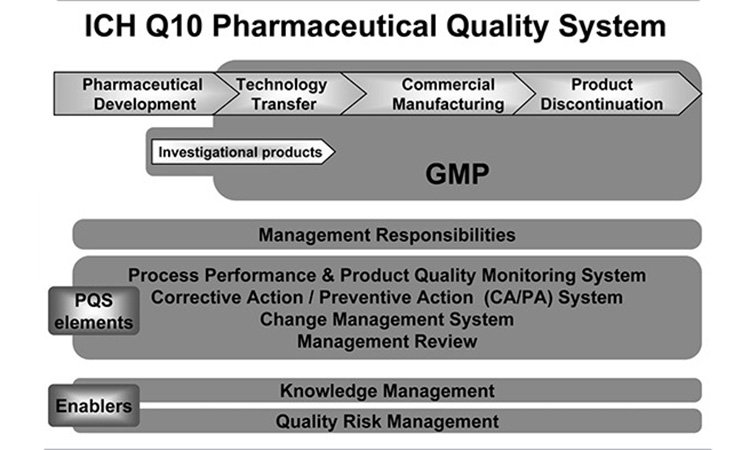
CAPA and Continuous Improvement using Process Performance & Product Quality Monitoring (PPPQMS), are elements of the Pharmaceutical Quality System (PQS), supported by ICH Q10. By practicing effective CAPA and PPPQMS a Pharmaceutical Quality System can realize Quality Management Maturity. Through lecture and “hands-on” team exercises this course illustrates how a mature Pharmaceutical Quality System utilizes CAPA and Continuous Improvement, how to effectively write and investigate to achieve holistic CAPA, and what PPPQMS can do for your PQS.
Learning Objectives:
ICH Q10, ICH Q7, 21CFR 211 regulatory framework and linkage to the Pharmaceutical Quality System
Understanding the importance of how CAPA and Continuous Improvement integrate with the Pharmaceutical Quality System
Understanding Quality System Management Maturity
- Where and how does Management Responsibility fit in
- What are the enablers of a Pharmaceutical Quality System and how they work
How to Perform Holistic and Effective Correction and CAPA
- Effective Deviation Authoring
- Investigation for True Root Cause
- Correction and CAPA
Product Performance and Product Quality Monitoring System (PPPQMS)
- Product & Process Understanding & Tools
- Monitoring and Continual Improvement
What You Will Learn
- To author an effective investigation
- Perform holistic correction and CAPA
- Reduce deviations through risk based continuous improvement
Resources and Activities
- Pre-Course Work Materials
- Interactive Exercises
- Learning Assessments
- APQ Guide: Corrective Action & Preventive Action (CAPA) System
- APQ Guide: Cultural Excellence
- APQ Guide: Management Responsibilities & Review (MRR)
- PQLI Guide: Part 4 - Process Performance & Product Quality Monitoring System
Who Should Attend
- Quality Assurance
- Tech Support
- Tech transfer functions
- Operations functions
- Early career (3-5 years within the industry)
- Qualified persons in Europe
- Team Lead/Operator/Technician Staff
- Managers
- Section heads
- Supervisors and CEOs
- President
- Owner or General Manager working in pharmaceutical and biotech manufacturing (not CMO)
- Engineering
- Architecture or construction
- Service provider or consulting
- Equipment or material supplier
Additional Course Information
Communities of Practice
This training course is of particular interest to existing and future members of the Regulatory and Quality Networking Communities.







