Technical
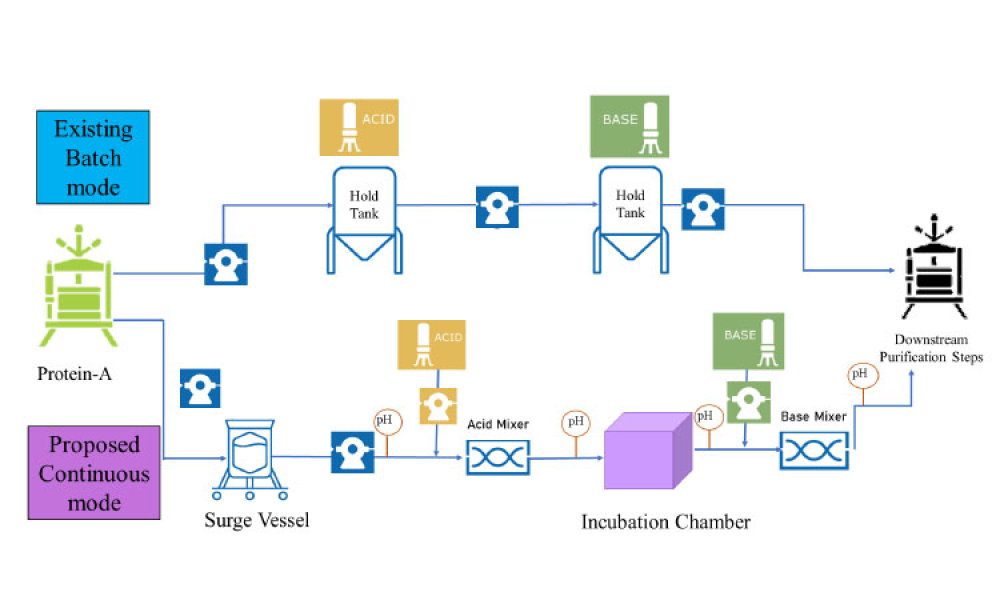
Viral inactivation (VI) is a critical step in ensuring the safety of monoclonal antibody (mAb), Fc fusion, and recombinant protein therapeutics and it is typically an important component of an overall virus control strategy for downstream biotherapeutic production processes. Considerations for successful implementation of an inline VI process are discussed in this article.