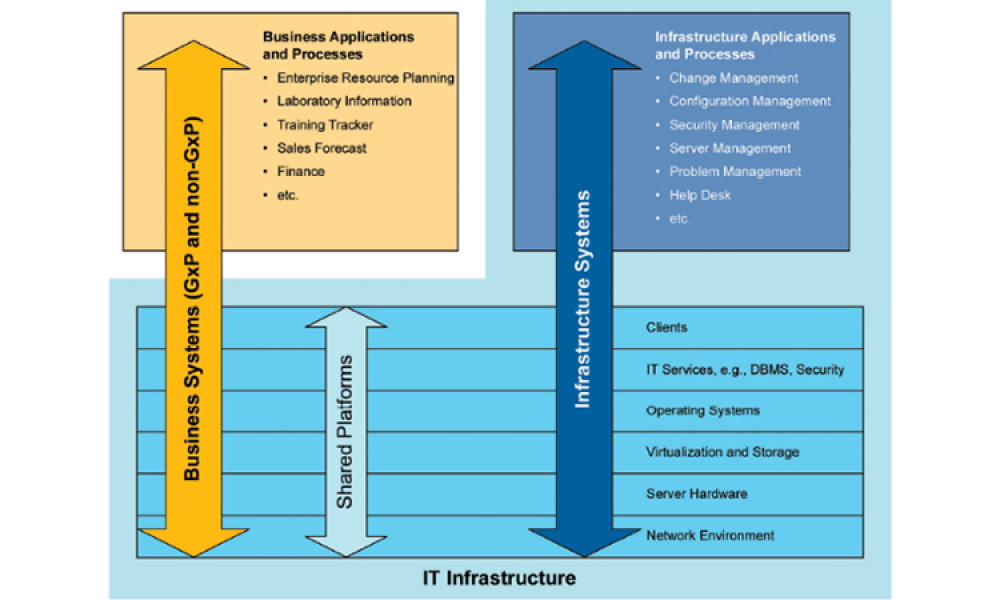
Features
In 2021, the ISPE GAMP® Community of Practice (CoP) is celebrating 30 years of promoting industry good practice for computerized systems and encouraging technical innovation and progress, while protecting patient...