Learning Level: Intermediate
Session Length: 3 hours
In this highly interactive session, attendees will have the opportunity to explore how the thoughtful integration of Quality Risk Management (QRM) and Knowledge Management (KM) can lead to more informed decision making across the Pharmaceutical Quality System (PQS) during technology transfer and commercial manufacturing. The discussion will be led by regulatory science researchers from TU Dublin and industry experts.
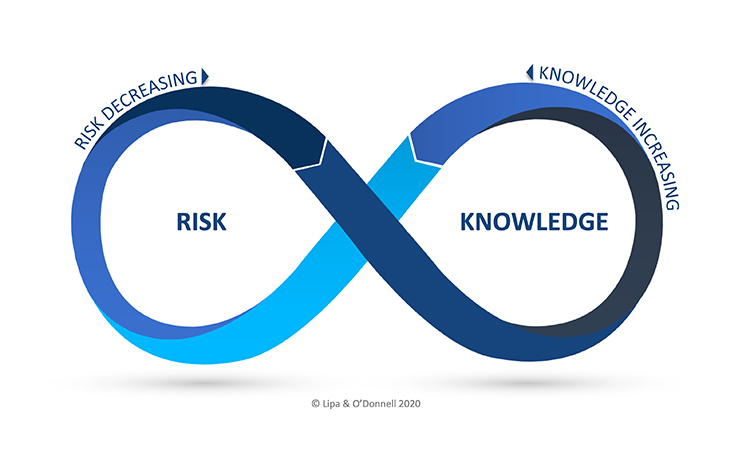
A novel framework to intentionally unite QRM and KM, the Risk-Knowledge Infinity Cycle (RKI Cycle), was first introduced in May 2021 through the ISPE Good Practice Guide on Knowledge Management in the Pharmaceutical Industry and an ISPE Webinar, Exploring the RKI Cycle Across the Product Lifecycle. The RKI Cycle will be presented in brief to orient the attendees, followed by a presentation on risk-based decision making. These presentations will be followed by breakout sessions to engage with the experts and fellow participants on how they view the potential opportunity and impact of the RKI Cycle, as well as potential implementation challenges.
First-hand Strategic Insights & Actionable Takeaways
- An introduction to the Risk-Knowledge Infinity Cycle (RKI Cycle) as a framework to unite QRM and KM
- An introduction to risk-based decision making (i.e., decisions based on science and evidence), and how the RKI Cycle can further enable risk-based decision making
- Preliminary considerations & opportunities for where the RKI Cycle may be deployed to drive more informed decisions for technology transfer and commercial manufacturing
- Download Knowledge Map Template
- Attendee Survey
ISPE Expert Xchange Session Speakers
Copyright Disclaimer
This copyright notice applies to all proprietary pages, images, text, programs, and other material available throughout this Internet site (collectively, this “Publication”). All materials posted on the website are subject to copyrights owned by the International Society for Pharmaceutical Engineering (“ISPE”) and other individuals or entities. Any reproduction, retransmission or republication of all or part of any documents found on this site is expressly prohibited, unless ISPE or the copyright owner of the material has expressly granted its prior written consent to reproduce, retransmit or republish the material. All other rights reserved. View entire copyright policy.
& Click the bell for notifications.



