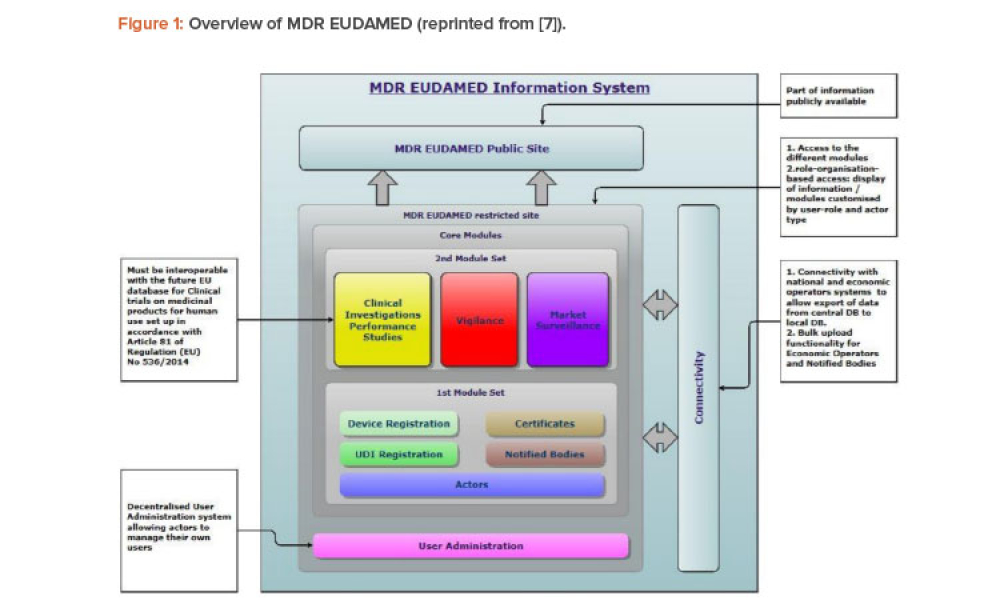
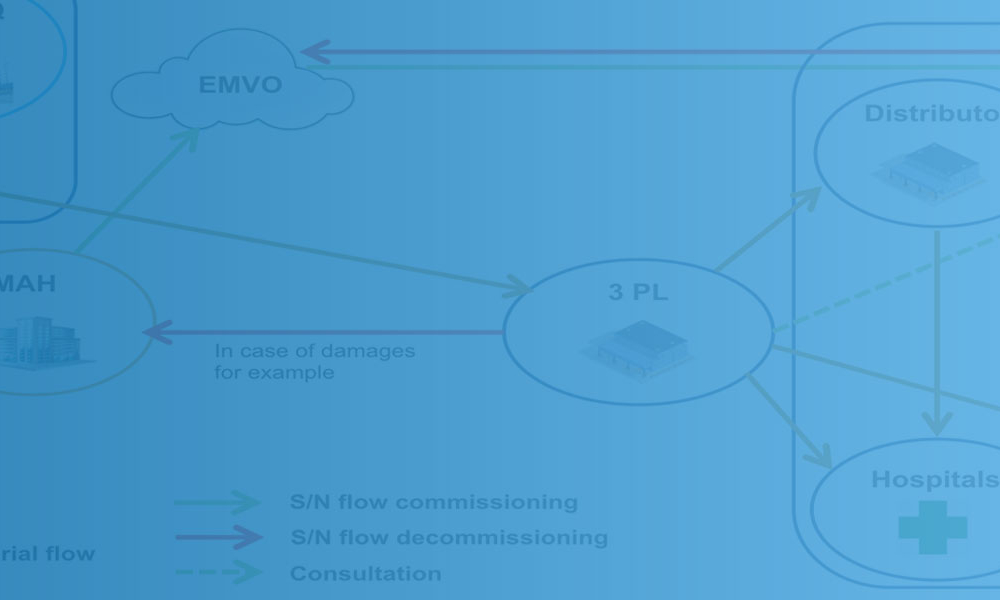
Technical
Since 2019, the ISPE France Affiliate’s Unique Device Identification (UDI) Medical Device Work Group has been producing tools to help project stakeholders within the EU or overseas understand and comply with EU regulations of UDIs in medical devices. Some of those tools are highlighted in the article.