The new ISPE GAMP® Guide: Artificial Intelligence provides a comprehensive, state-of-the-art best practice framework to efficiently and effectively achieve high-quality AI-enabled computerized systems in regulated life science areas. It bridges established GAMP concepts with...
Martin Heitmann

Related Articles
The landscape of clinical trials has been transformed in a post-pandemic world. In July, ISPE released the second edition of ISPE GAMP® Good Practice Guide: Validation and Compliance of Computerized GCP Systems and Data – Good eClinical Practice. It addresses managing complexities associated with decentralized clinical trials, the benefits and challenges of using open-source software, and...
Stakeholders across industries are becoming accustomed to using information technology (IT) systems, applications, and business solutions that feature artificial intelligence (AI) and machine learning (ML). Even though some of these uses show phenomenal performance, thorough risk management is required to ensure quality and regulatory compliance are met within the life sciences industry. By...
ChatGPT and other large language models are positioned to change the world. They can also shift acceptance and prevalence of machine learning solutions in regulated industries in general. However, their arrival requires reconsiderations on risks, quality assurance, and validation from a GxP perspective.
Recent advances in artificial intelligence (AI) have led to its widespread industrial adoption, with machine learning (ML) algorithms demonstrating advances in performance in a wide range of tasks. However, this comes with an ever-increasing complexity of the algorithms used, rendering such systems more difficult to explain.
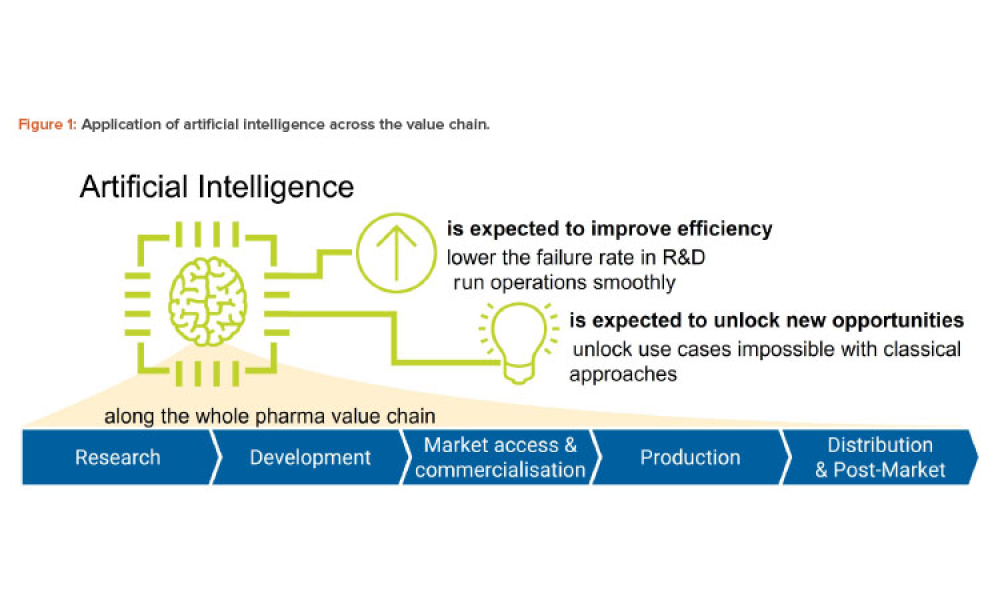
Artificial intelligence (AI) has the potential to benefit the pharmaceutical industry and its GxP-regulated areas. Several pharmaceutical companies are currently running digital pilots; 90% of large pharmaceutical companies have initiated AI projects.