Features
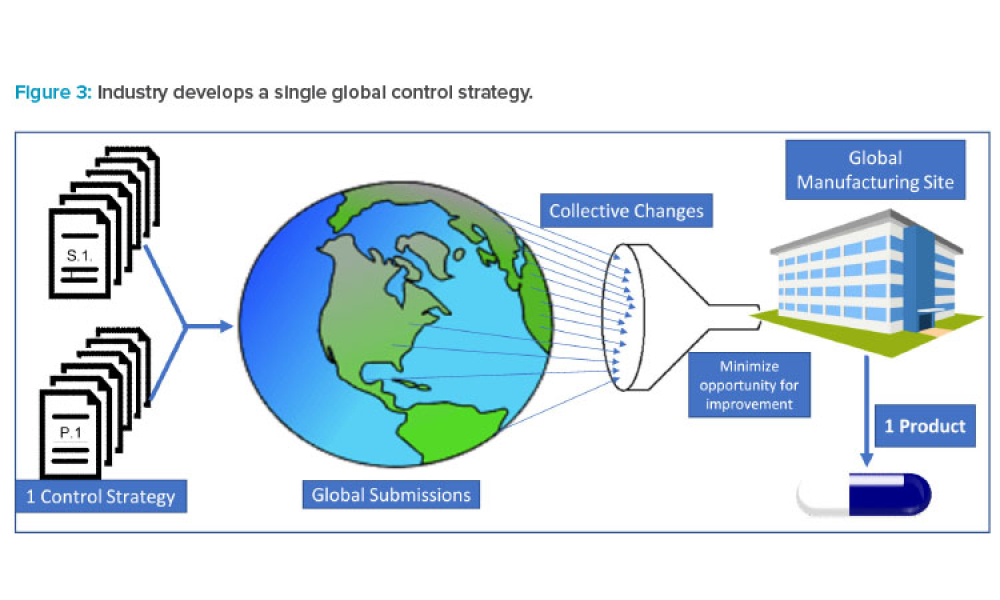
During the past decade, industry has experienced a proliferation of regulatory divergence regarding the interpretation and implementation of International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines (and control strategies) across geographic regions. This article shares data that highlight instances where well-established ICH...