Features
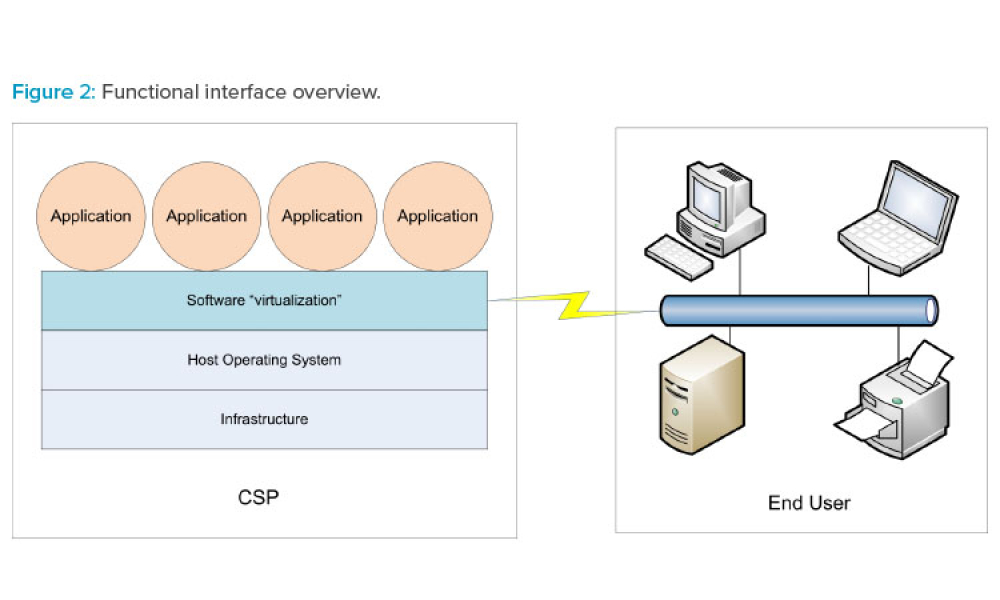
Cloud computing can be described as networked access and utilization of configurable computing resources such as data and information storage, processing capabilities, applications, and other services on computerized systems provided and/or maintained by a remote organization. As life sciences companies consider the advantages and costs of utilizing cloud services, they first need to invest...