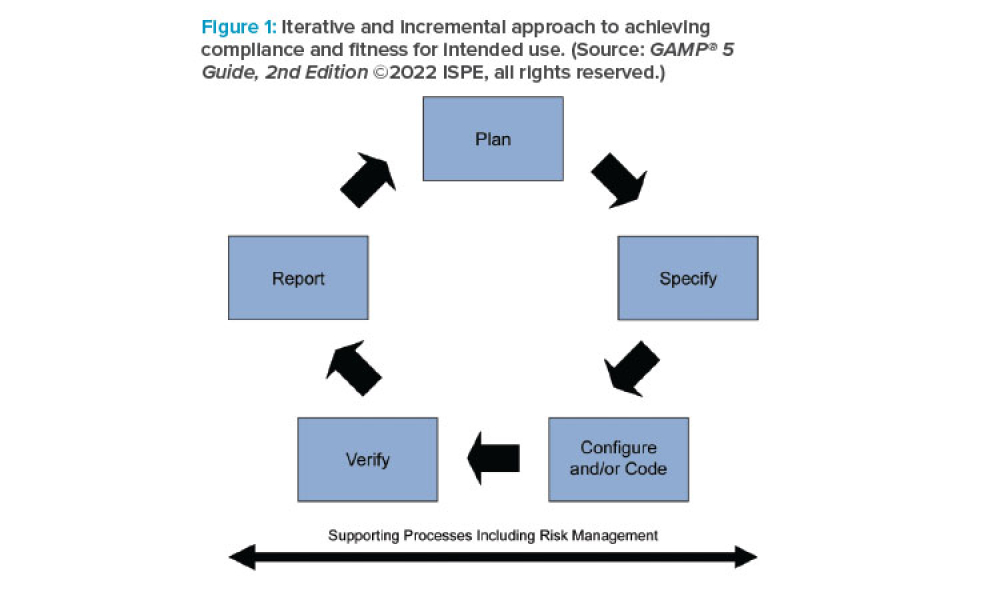
ISPE’s GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems (Second Edition) (GAMP® 5 Guide, 2nd Edition) maintains the principles and framework of the first edition and updates their application in the modern world, including the increased importance of service providers, evolving approaches to software development, and expanded use of software tools and...
Features