Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
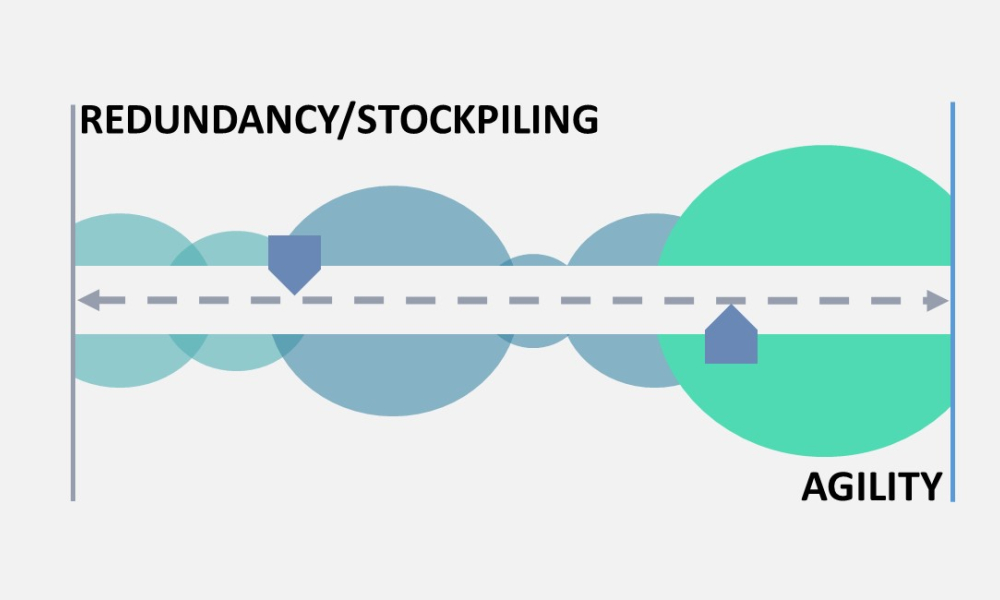
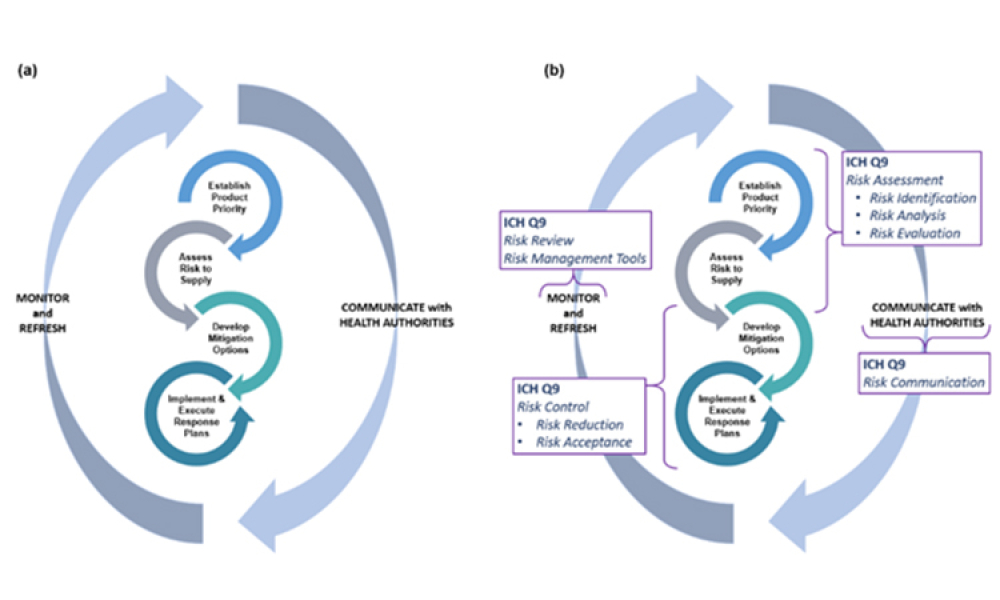
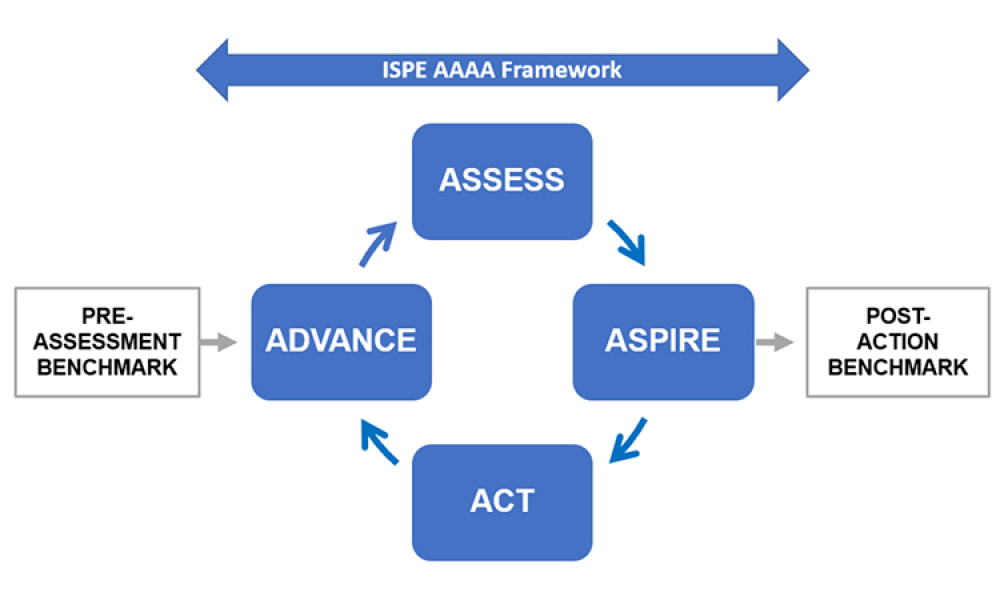
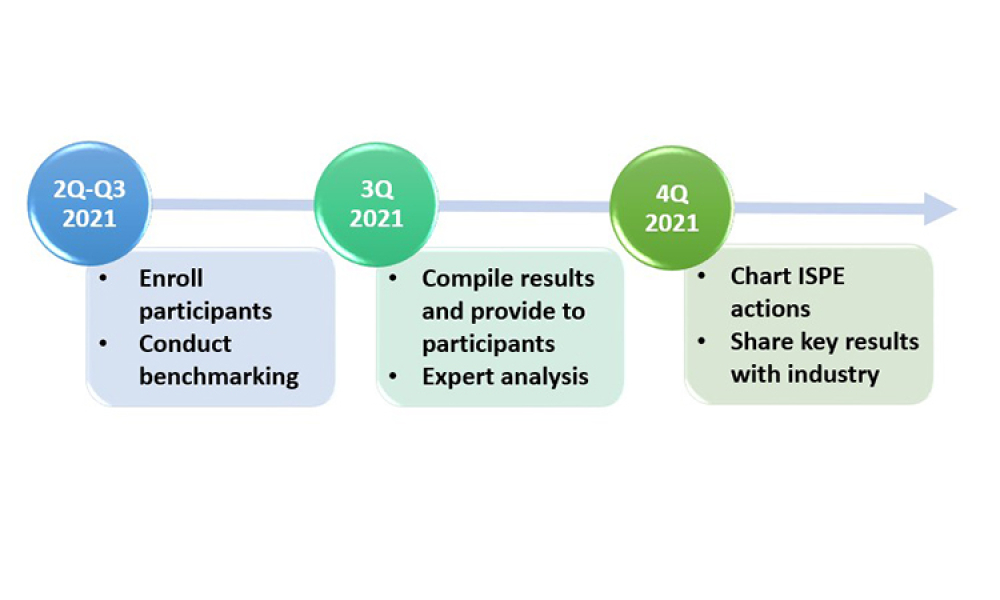
ISPE’s Drug Shortages Initiative focuses on the technical, scientific, manufacturing, quality and compliance issues associated with a company’s supply chain and related to its ability to source, manufacture and distribute products that have resulted in drug shortages.
Any effort to effectively address the complex and multi-faceted issues contributing to drug shortages requires close technical collaboration and clear communication between the pharmaceutical industry and global health authorities. For nearly a decade, ISPE has been instrumental in facilitating communication between the different sectors of the pharmaceutical industry and global health authorities related to drug shortages.
Guidance Documents
Active Pharmaceutical Ingredients (1)
+Drug Shortages (3)
+Project Management (1)
+Quality Assurance (1)
+Regulatory (2)
+Supply Chain Management (1)
+Community Discussions
Community Discussions
Apr 16, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Apr 07, 2025
Advanced Manufacturing
Artificial Intelligence
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
Featured Conferences