November / December 2020
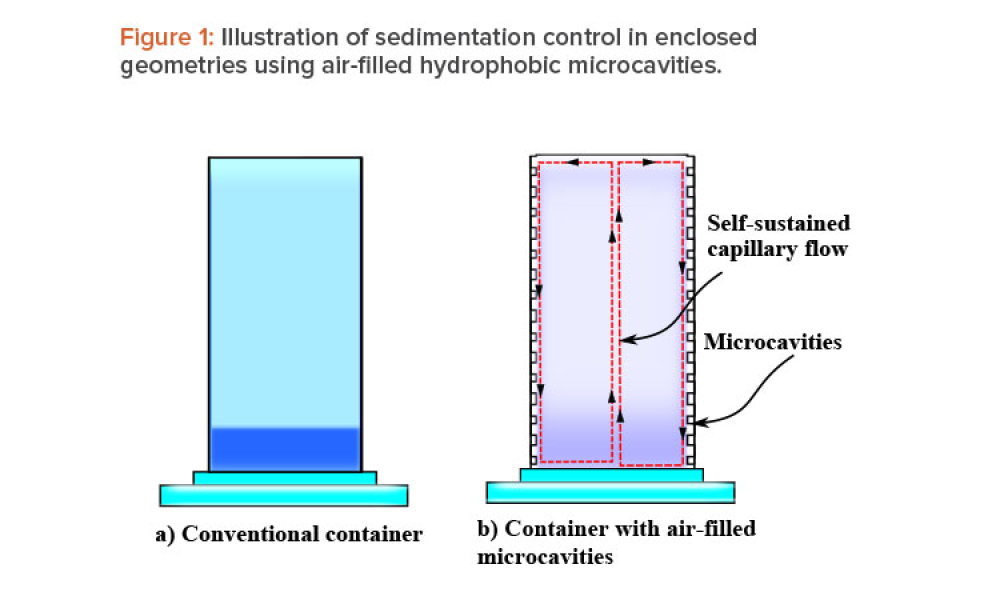
When fluid-filled containers are stored for long periods of time with negligible motion, sedimentation and gravitational settling of particles can occur. This article discusses a passive sedimentation control mechanism that is driven by Marangoni stress, which is induced in enclosed geometries when their walls are lined with gas-filled hydrophobic microcavities.
Executives at manufacturing companies of all sizes need to make decisions about where to invest to maintain and grow their businesses. Investments in manufacturing execution system (MES) applications may reduce costs and increase revenues, but they also might compete with other investment priorities, such as marketing campaigns and capital equipment upgrades. This article offers guidance for...
To continue meeting the professional needs of ISPE members and with the global increase of virtual events due to COVID, ISPE has expanded its webinar offerings on topics important to ISPE members and industry sponsors.
ISPE is celebrating its 40th anniversary this year! Here are some of the memorable events from the last 40 years.
The ISPE D/A/CH affiliate Is a Leader in Many Respects, from Its Ever-Increasing Size to the Invaluable Engagement of Its Members, Who Contribute to Ispe’s Growth around the World. Grouping Together Europe’s German-Speaking Countries—Germany (D), Austria (a), and Switzerland (Ch)—It Is Currently the Second Largest Ispe...
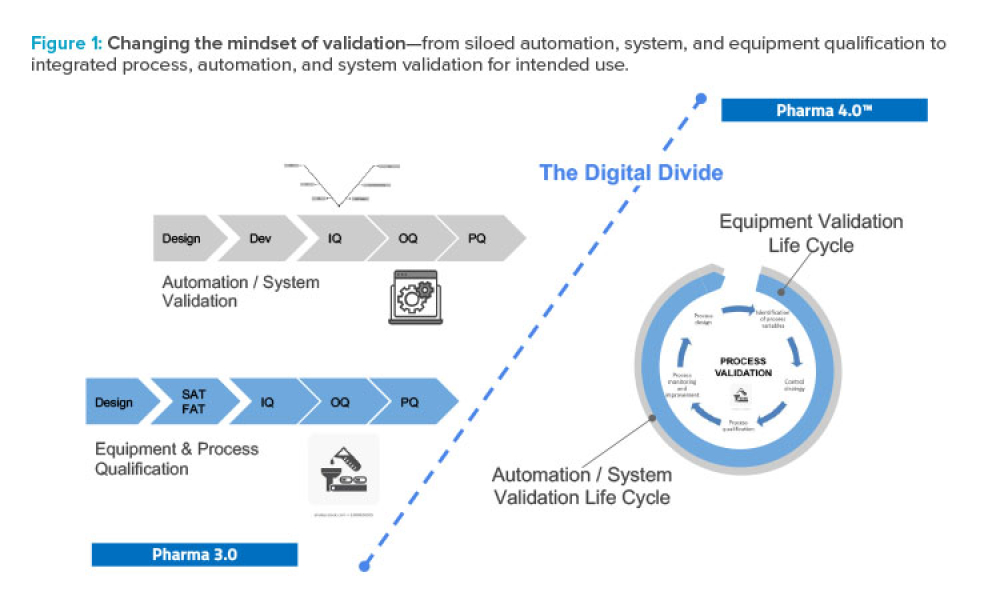
As Pharma 4.0™ increasingly becomes reality, our validation practices must change. We can no longer apply 20th-century thinking to 21st-century technology and resources. Validation must adapt to industry shifts from iterative to...
Joydeep Ganguly, who is currently Senior Vice President, Corporate Operations, at Gilead Sciences, Inc., places a premium on working for a mission-oriented company. “If you choose the right company with the right ethos and a deep, tangible commitment to the patients, the mission is not just rhetoric,” he said. “You see it in the way they do things. If you work for a company that makes that...
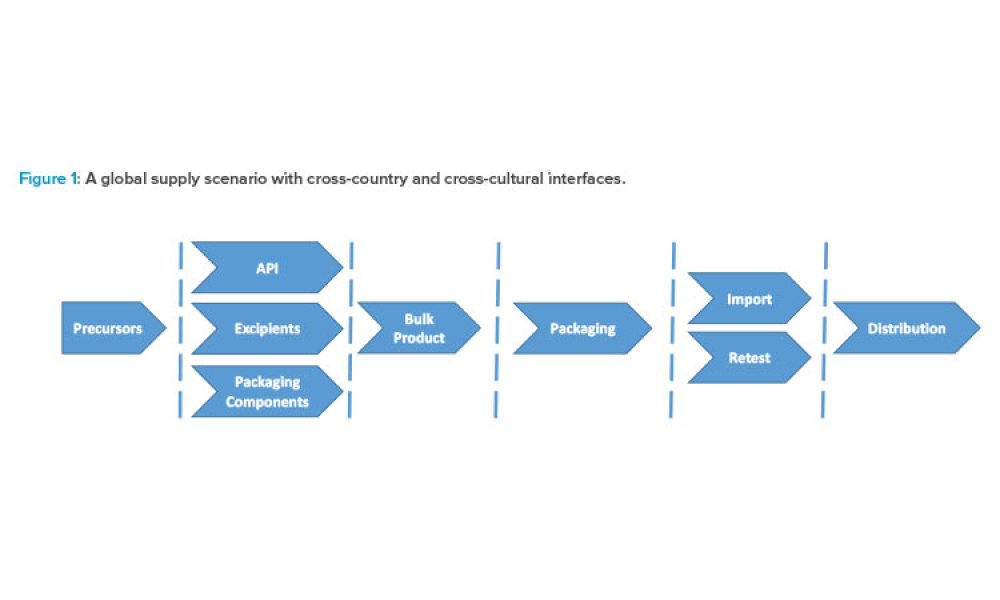
The current regulatory framework in the pharmaceutical industry places pressure on marketing authorization holders (MAHs) to demonstrate quality oversight, and a systematic risk management process is a prerequisite for avoiding compliance and productivity pitfalls. This article focuses on options to improve day to- day operations and to ease decision-making by integrating operational risk...
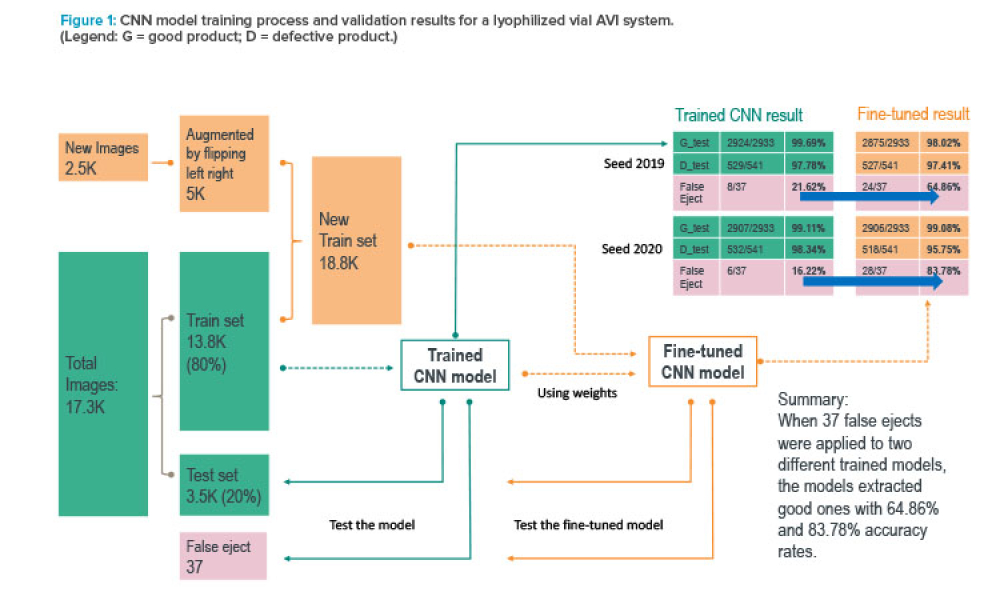
Pharmaceutical companies rely on automated vision inspection (AVI) systems to help ensure product safety. Although these systems overcome challenges associated with manual inspection, they can be hindered by limitations in their programming—if the system is programmed to consider every variation in inspection conditions, it is likely to falsely identify defects in safe products. This article...