Challenges & Results: Moving to Multicolumn Chromatography

Although pharmaceutical manufacturing is traditionally a change-averse industry, the benefits of continuous processing demonstrated in other industries are beginning to drive interest in its application to bioprocessing. This article reports on MedImmune’s exploration of bioprocess areas that could be advantageously transitioned from batch operations to continuous processing.
Downstream processing is often the most challenging part of any bioprocess, and chromatography is a cost-and time-intensive portion of this operation due to the complex nature of the technology and the high cost of the sorbents (also commonly referred to as resins) required. As such, we evaluated continuous multicolumn chromatography (MCC) for its potential to reduce costs and deliver other benefits (lower sorbent consumption, flexible footprints, etc.) at large scale.
Process Intensification
Mature industries such as car manufacturing, steel production, and commodity chemical manufacturing have established continuous processing solutions over the past 100 years. This has helped improve capital utilization and reduce operating expenses while achieving greater process control, which enhances safety and product quality.
When applied to the production of biopharmaceuticals, continuous processing enables similar benefits. While buffers, sorbents, raw materials, viral clearance fundamentals, bioburden-control systems, contact materials, and other attributes of batch processing do not change after conversion to continuous manufacturing, overall productivity improves, with higher and more consistent yields. Greater process control enhances product quality. And since more productive continuous bioprocess systems tend to be more compact, current manufacturing footprint can be conserved or reduced.
In addition, because batch scale-up is replaced with increasing process run times, continuous processing provides a greater ability to respond to variable market demand. Adopting single-use technologies further increases efficiencies—with faster process setup and elimination of cleaning/cleaning validation steps—while reducing the risk of cross-contamination in multiproduct facilities.
Stepwise Transformation
Moving from batch manufacturing to a fully integrated continuous process involves significant change and investment, both of which carry an elevated level of risk. Recognizing the value of the continuous processing approach while understanding the evolution is just beginning.∗
Because of this, MedImmune has approached continuous bioprocessing for unit operations by adopting a modular approach that implements continuous manufacturing where it offers the greatest benefits. This hybrid approach allows us to gain experience in the continuous processing space while continuing to maximize the value of our existing batch manufacturing infrastructure.
Multicolumn Chromatography
The first phase in this modular approach explored MCC as an alternative to batch chromatography for protein A capture. Chromatography is a fundamental unit operation in the purification process for which alternative solutions may offer economic and efficiency advantages.
In typical batch primary capture chromatography, less than 60%–70% of total sorbent binding capacity is used. As bioprocess fluid is fed to the column, the top sorbent becomes saturated but the bottom encounters little to no product. All activity occurs in the mass-transfer zone, which accounts for only a small portion of the column, while the remaining areas serve as idle zones.
Saturating the latter portion of the sorbent would result in costly product breakthrough, so initial product development experimentation sets a binding capacity limit of 10% product breakthrough. As this value propagates into the manufacturing setting, however, additional safety factors are added, further reducing the resin utilization. Higher binding capacities could be attained by increasing the residence time of the bioprocess fluid on the column, but this would affect processing time and total time in plant. In traditional batch mode, sorbent underutilization is the manufacturer’s opportunity cost that is sacrificed to strike a balance between speed and product recovery.
* Some downstream processing steps (i.e., filtration and flow-through chromatography) are currently compatible with continuous bioprocessing, and there is extensive discussion in the pharmaceutical industry about developing fully continuous end-to-end, integrated downstream bioprocessing solutions.
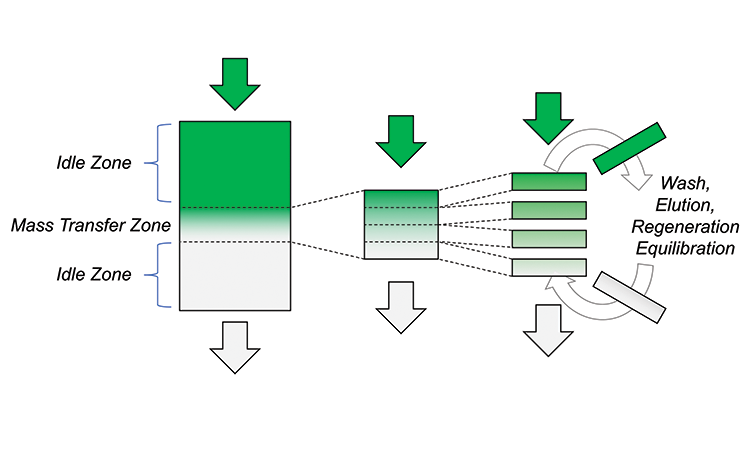
With MCC (Figure 1), smaller columns with reduced bed heights are linked in series and used for the same process steps that a batch column would undergo. These smaller columns are cycled multiple times to process a comparable volume. In this case, however, the resin is allowed to load to high breakthrough, with product from the first column captured on the second (second pass) column. This preserves high yield, but also exploits a much higher binding capacity on the first column. With continuous countercurrent loading, the same residence times are achieved, but only the mass transfer zone must be accommodated by the load columns, eliminating the sorbent required for idle zones in batch chromatography.
Much less sorbent and much smaller columns, therefore, purify the same amount of product as in a batch process. Overall, greater than 40%-higher sorbent capacity can be attained. A batch column, for example, typically achieves a binding capacity of 35 grams (g) of product per liter (L) of sorbent (at 10% breakthrough). Continuous processing could achieve a binding capacity of 50 g/L or more. Greater than 95% of equilibrium binding capacity can usually be attained, a parameter that is simply unattainable in batch processing. In addition, no product is lost to breakthrough, and buffer requirements are reduced. Furthermore, due to rapid cycling, the multicolumn configuration can be designed to allow chromatographic sorbent to reach its full reusable lifetime within a campaign, allowing a single-use/disposable format. Importantly, all batch process steps are maintained, and the same sorbent chemistries and buffer solutions can be used.
Business Case
To compare continuous and batch processes, the first step in our exploration was to calculate potential cost savings at large scale for different column sizes. This translates the benefits of the higher binding capacity and smaller columns into tangible criteria, such as time in plant and cost of goods.
For this exercise, a 2,000-L batch with a titer of 5 g/L monoclonal antibody (mAb) was processed in batch mode using a column with a height of 20 centimeters (cm) and a 60-cm diameter, or in continuous mode using either four columns with a height of 10 cm and a 30-cm diameter, or three columns with the same height but a 20-cm diameter.
For the batch process, 57 L of sorbent and six cycles requiring 6,100 L of buffer and a total column wet time of 8 hours (hr) afforded a productivity of 20 g/L sorbent/hr. For the continuous process designed with comparable processing time, eight cycles were performed, reducing the sorbent (28 L), buffer (4,000 L), and column wet time (6 hr). With the smaller column MCC design, the number of cycles and wet time were notably higher (25 and 20 hr, respectively) than the batch process. Sorbent requirement, however, was dramatically reduced to 9 L, and buffer consumption was lower still at 4,200 L.
Importantly, both continuous processes had measurably higher productivities: 60 and 55 g/L sorbent/hr for the larger and smaller columns, respectively. Cost savings were also significant: Assuming a cost of $12,000/L of sorbent, the batch process had a total cost of $648,000 compared with $336,000 and $108,000 for the two continuous operations.
In the first continuous process with 30-cm columns, buffer and sorbent consumption and column wet time are all reduced, tripling productivity as measured in g/L sorbent/hr. This scenario is advantageous when converting an existing batch process to a continuous operation. Run times for the chromatography process are already established and fit into the overall downstream operations. Any process time extension in a unit operation will affect the scheduling of operations that follow. Using a larger column allows for a wet time similar to the batch process, meeting the time constraints as defined by the existing batch process.
In the second continuous scenario, using smaller columns tripled the column wet time compared to the batch process. This approach might not, therefore, be suitable as a replacement for an existing batch operation. On the other hand, sorbent consumption is markedly lower, leading to a cost that is one-third that of the first continuous process and nearly one-sixth that of the batch process. This would be attractive when a plant is underutilized or where the costs of goods is more important, such as in a clinical production facility. Similar calculations were then performed for chromatography at the 15,000-L scale using a bioprocess fluid with a mAb titer of 5 g/L. In this case, the batch process was conducted using a column with a height of 20 cm and a diameter of 1.8 meters (m), while the continuous process was carried out once again using four columns with a height of 10 cm and a diameter of 30 cm. In this case, going from batch to continuous processing, consumption was reduced from 509 L to 28 L. Although the process time nearly doubled— going from 24 to 40 hr—productivity increased more than tenfold from 5 to 56 g/L sorbent/hr, and the cost was reduced by a factor of nearly 20, from $6,108,000 to $336,000.
These results demonstrate that switching from batch to continuous processes can dramatically reduce the cost of goods for biopharmaceutical chromatography processes. They also reveal several ways in which benefits can be realized with MCC technology, depending on plant constraints and the company’s goals. Options exist for sorbent, buffer, and time savings, and process engineers can design continuous solutions to meet the demands of any project.
Additional Benefits
The smaller quantities of sorbent and buffer required for MCC lead to other benefits as well. Lower inventory and storage requirements for smaller amounts of sorbent and buffers reduce costs and improve balance sheets. Sorbent savings are also realized for clinical programs that do not move forward.
Risks diminish as well. Because the sorbent lifetime could be consumed with a single campaign, for instance, there is no need for sorbent storage, reducing the risk of contamination over successive batches, storage, and campaigns. Smaller columns also improve safety.

Finally, MCC integrates seamlessly with upstream continuous processes. When a company is ready to move toward end-to-end continuous processing, perfusion reactions can be readily coupled with downstream chromatography operations.
Drivers
MedImmune’s main driver for exploring continuous chromatography was the potential for sorbent savings, regardless of whether MCC is coupled with other batch operations or incorporated into a fully continuous process. The company was particularly interested in potential savings for protein A capture chromatography, because protein A is the most expensive sorbent used at MedImmune.
Once the calculations indicated that MCC was worth exploring in the lab, the next step was to select a continuous chromatography system. MCC has been around for many years, but until recently was not suitable for use in good manufacturing practice environments for biologic drug substances. The systems require extensive plumbing, which previously presented impractical setup, cleaning, and maintenance challenges.
MedImmune wanted a continuous chromatography system that could be set up and operated readily, and one that would allow an easy transition from batch to continuous processing. It needed a system flexible enough to operate processes from small to large scale, with varying numbers and sizes of columns. The company expected to investigate low-titer bioprocess fluids generated in perfusion bioreactors, higher-titer fluids from fed-batch reactions, and concentrated batch harvests with titers up to 15 g/L. To enable scale-up comparisons, a system with a higher throughput and the ability to use columns from 5 milliliters (mL) to 100 mL in size was also crucial.
The Cadence BioSMB platform (Figure 2) from Pall Life Sciences met these requirements. The system is based on a single-use, integrated valve block design with 240 diaphragm valves. One valve cassette is used per campaign. The system is available at both process-development (16 columns) and production scales (8 columns) with 1- and 3-millimeter flow paths that can operate at rates up to 70 mL/min and 5 cm columns, or 300 mL/min and columns up to 10 cm, respectively.
Finally, we used an effective modeling approach that allows easy conversion of batch processes to continuous operations. The process model enables users to quickly convert single-column breakthrough data for a batch process to the parameters appropriate for a continuous process. Notably, the same sorbent, buffer system, and product quality assays can be used.
Protein A Case Studies
Once the MCC system had been installed, we performed both small- and larger-scale continuous chromatography runs, then compared the results to those obtained from similar batch processes, using a clarified media containing immunoglobulin G mAb to investigate protein A capture chromatography.
The batch process used 20 mL of sorbent. Cycle time was 2.5 hr, with 650 milligrams (mg) of product processed per cycle, leading to a productivity of 13 g/L sorbent/hr.
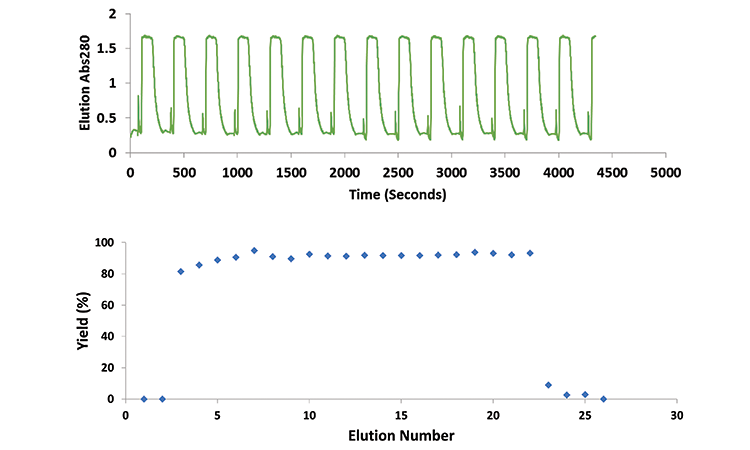
The continuous process used 25 mL of sorbent, but cycle time was reduced to 48 minutes (one-fifth that of the batch cycle). A total of 820 mg of product was processed per cycle once steady-state conditions were achieved (ramp-up occurred over the first two cycles). This yielded a productivity of 40 g /L sorbent/hr—a nearly four-fold increase.
Figures 3 and 4 show the small-scale continuous process performance. Steady-state yields were 91.0% ± 1.5%, and the total process yield was 92%. The repetitive elution peaks could allow cycle-to-cycle overlay for multivariate analysis, which could enable dynamic monitoring and provide insight into aging sorbent characteristics (Figure 3).
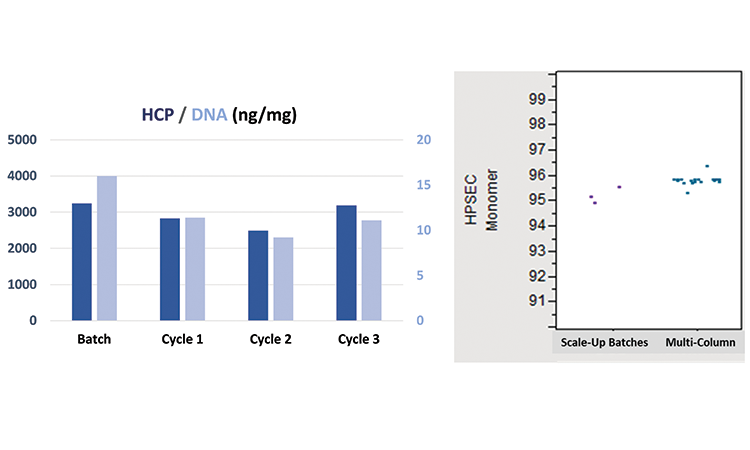
Host-cell protein and DNA impurities in the final product eluates were slightly higher in the batch process than in the continuous process steady-state cycles 1, 2, and 3 (Figure 4). Results are on the same magnitude, however, and can be considered similar.
The second graph in Figure 4 plots the ratio of monomer to aggregates for different runs. The scale-up batch data is historical data collected for several scale-up (50–200 L) chromatographic purifications of the same mAb purified in the continuous run. Multicolumn data points for various cycles are clustered at a higher purity (the two outliers are associated with the ramp-up and ramp-down cycles). The tighter purity profile is the most important information obtained here. This study confirmed product quality comparability to batch processing.
Productivity At 50-l Scale
After evaluating the continuous MCC using 5-mL columns, we conducted a second investigation using ±100 mL of sorbent in larger columns (4.4 or 5 cm). This was an internal check to confirm that quality and yield results comparable to existing batch processes could be obtained with continuous multicolumn chromatography at mid-scale.
The MCC results were compared with batch runs at the 50–200-L scale for a variety of mAbs (platform, legacy, bispecific, fusion). No large variations or differences were seen. While product quality and yield advantages were not observed in all cases, sorbent, buffer, and time savings were achieved.
In one example, a bioprocess fluid with a mAb titer of 4.3 g/L was purified in both batch and continuous modes. In the batch process, a column with a height of 20 cm and a diameter of 14 cm required 3.1 L of sorbent (at a cost of $37,000) and 200 L of buffer. Three cycles were completed in 6 hr, resulting in a productivity of 12 g/L sorbent/hr.
For the continuous process, MedImmune wanted to use the smallest amount of sorbent possible. Four columns with a height of 5 cm and a diameter of 4.4 cm were used, requiring 300 mL of sorbent ($3,600) and 100 L of buffer. Fourteen cycles were completed in 11 hr, affording a productivity of 60 g/L sorbent/hr. Although the process took nearly twice as long, productivity was boosted by a factor of five, at one-tenth the cost and half the buffer.
Conclusions
MCC has significant potential to improve the cost and efficiency of chromatography processes in biopharmaceutical manufacturing. These potential benefits were significant enough that MedImmune conducted modeling and physical studies to explore continuous MCC in its production facilities.
In multiple protein A purifications of different mAb products at different scales (3–50 L) using a range of column sizes, product quality between batch and continuous runs was consistent. We observed increased binding capacities, reduced buffer requirements, reduced sorbent requirements, and improved productivity results, using the same batch process steps and development approach.
Based on these results, MedImmune will explore continuous MCC to improve other chromatographic separations, including the possibility of using higher-cost, higher-capacity sorbents that are impractical for batch processes. Using prepacked columns in combination with other existing single-use technologies could further facilitate continuous chromatography setup and operation in an entirely disposable approach.
The flexible throughput capabilities of continuous MCC should also be explored in diverse types of facilities for the potential to address problems ranging from the need for lower cost of goods to reduced buffer consumption due to lack of space, and many others.
Finally, the positive results obtained for MCC bring MedImmune one step closer to full continuous processing. As we gain additional experience, we will continue to target continuous unit operation implementation where the greatest benefits can be realized.