I am pleased to have another opportunity to share some thoughts and provide a quick update about the progress of ISPE. It is hard to fathom that we are halfway through 2018, and summer is upon us (at least those of us in the Northern Hemisphere). ISPE had a very busy first half of the year, with three major conferences in North America, a very successful Europe Annual Meeting in Rome, and...

Downloads
The Fourth Industrial Revolution
Cover: Technology is advancing faster than ever. Rapid, major changes are on the horizon for just about everything—including manufacturing. Connectivity and artificial intelligence, along with flexible automation, will allow us to move from a reactive shop floor to an autonomous, self-organizing factory.
Digital Labels Revolutionize IMPs
Feature: Digital technology is helping clinical trials find new ways to incorporate faster and lower-cost labeling options for IMPs. e-Labels can contain a regulatory-compliant IMP label, and they may also decrease or even eliminate deviations caused by extension relabeling, sterility breaches, tamper-evident seal removal, product mix-up, and temperature excursions. In addition, IMP changes that affect label content can be communicated quickly.
Europe Annual Conference
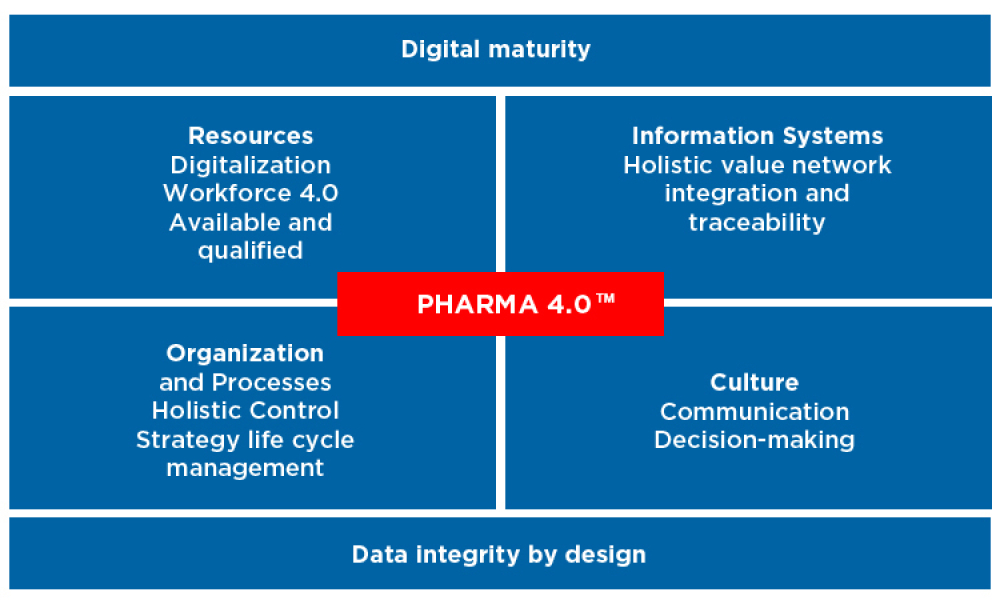
Special Report: In March 2018, more than 640 attendees met in Rome for the 2018 Europe Annual Conference. The record attendance and roster of 70 speakers made it the largest ISPE Europe meeting to date. We cover the keynotes, Pharma 4.0, the YP Hackathon, and other highlights.
In This Issue
Recent cyberattacks like WannaCry and Petya have affected GxP computerized systems, prompting questions on how to address risk from cyberspace using traditional computerized systems validation according to GAMP® 5. This article explores life cycle management of GxP computerized systems and associated cybersecurity risks that can affect patient safety.
Double-wall containment: an answer to unsafe piping systems
While most in the pharmaceutical industry understand the need for double-wall containment piping systems, our field observations indicate that many companies do not. We frequently see pipes that should be (but are not) double-wall containment systems. This article presents an overview of the topic, so that readers who...
Change often takes years longer in the pharmaceutical industry than in others. Why can we not challenge that paradigm? The auto industry, for example, successfully forced changes to its supplier base within a couple of years. By benchmarking other industries that have dealt with similar problems, we can learn from their experiences.
This year's Europe Annual Meeting in Rome featured YP track leads, YP keynote speakers Caroline Rocks and Robert Landertingera.
In March 2018, more than 640 attendees met in Rome for the 2018 Europe Annual Conference.
For the jobs of tomorrow, STEM fields—science, technology, engineering, and mathematics—are powerful economic competitiveness drivers: 80% of the fastest-growing occupations depend upon mastery of math and science, and 92% of traditional STEM jobs require at least some post-secondary education and training. But in the United States, almost 50% of students have lost interest in the...
I finally landed a great job. How can I get started on the right foot?
Labeling is an important part of the supply chain. This is especially true for investigational medicinal products (IMPs), which must be labeled with clear expiry dates and other mandated information. IMP shelf life is notoriously difficult to quantify, however, and new findings on their stability frequently emerge during clinical trials. This often requires companies to relabel IMPs with...
The digital revolution is driving change across all industries. With its ability to increase transparency and trust between parties, the recent innovation called blockchain has the potential to significantly disrupt the clinical trials industry.
Gain complimentary access to select ISPE Good Practice Guides through the new portal. Members appreciate the practical knowledge and real-world examples built into our Good Practice Guides, so we made them available for free on the Portal.
My road to becoming an ISPE chapter president started by joining a student chapter.
Dr. Enno de Boer says rapid, major changes are on the horizon for just about everything—including manufacturing.
Chapter President Nicki Lange is cultivating an enthusiastic membership and building a professional community.
My conversation with six members of ISPE's Board of Directors continues this month.
In our industry, as in many others, the most important goal is developing and delivering a product that satisfies our customers. In our industry, the end customer is the patient.