Pharmaceutical clean (pure) steam systems consist of a generator, distribution tubing or piping, thermodynamic or balanced pressure thermostatic traps, control valves, pressure- reducing regulators, pressure gauges, pressure-relief valves, and volumetric totalizers. Most of these components are made of 316L stainless steel and contain fluoropolymer gaskets (most commonly polytetrafluoroethylene, also known as PTFE or Teflon), as well as semimetallic or other elastomeric materials. These components tend to corrode or degrade in service, potentially compromising the quality of the final clean steam (CS) utility product.
This project investigated stainless steel coupon samples from four CS system case studies, testing condensate for metals and particles, and conducting a risk assessment of potential corrosion effects on process and critical utility systems. Examining the corrosion byproducts involved preparing sample coupons of corroded tubing and components from distribution systems.
These case studies investigated a variety of surface conditions, and included analysis of typical rouge products and corrosion effects. The referenced sample surfaces were evaluated for rouge deposits by visual inspection, scanning electron microscopy (SEM), auger electron spectroscopy (AES), and electron spectroscopy for chemical analysis (ESCA)/x-ray photoelectron spectroscopy (XPS). These techniques reveal the physical and atomic properties of the corrosion and deposits, and identify potential contributions to the critical utility fluid properties or final product.
Overview
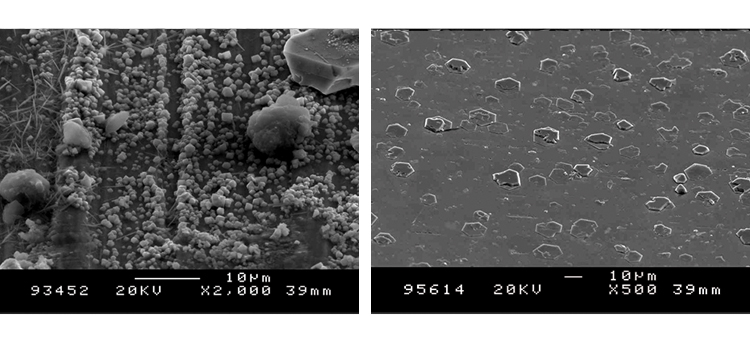
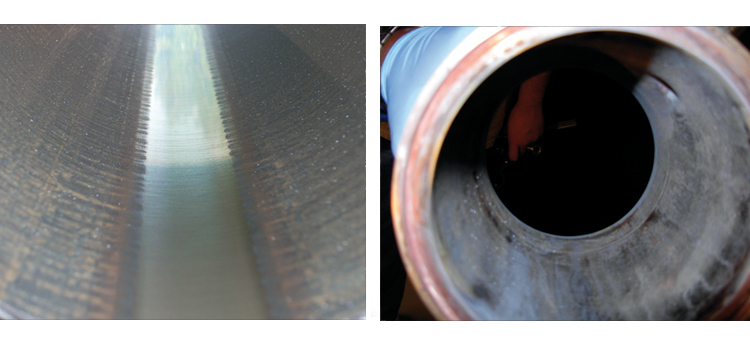
Stainless steel corrosion products are encountered in a variety of forms, such as a ferric oxide rouge layer (red or brown) on the metal surface found under- or overlying the thicker ferrous oxide layer (dark gray or black).
The rouge layer is crystalline in structure and potentially dynamic, or capable of migrating downstream. The ferrous oxide (black rouge) layer tends to thicken over time as the deposit becomes more pronounced; its migratory presence is evidenced by particles or deposits found on sterilizer chamber surfaces and on equipment or vessels after steam sterilization. Laboratory analyses of condensate samples illustrate the particulate nature of the rouge as well as the level of soluble metals in the CS fluid.
Water quality plays a major part in rouge product chemistry
While there are multiple causes of these phenomena, the CS generator is often a significant contributor. It is not uncommon to notice ferric oxides of rouge (red/brown) on the surface, with ferrous oxides (gray/black) at the steam discharge, with both types slowly migrating throughout the CS distribution system.
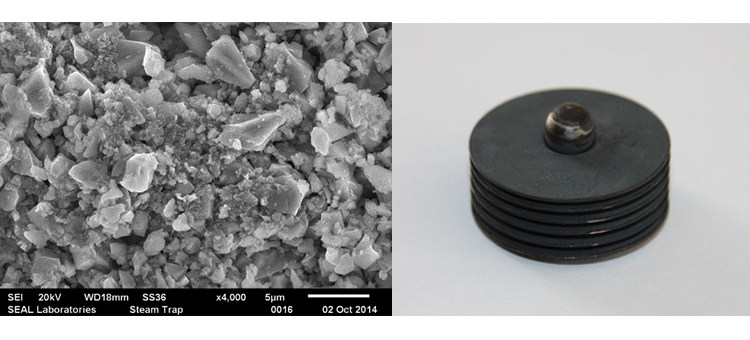
The CS distribution system is a branching configuration that has multiple use points, terminating at distant areas or ends of a main header and various branching subheaders. The system may include a series of regulators to reduce pressure/temperature at certain use points; these may be sites for corrosion. Corrosion can also occur in hygienically designed traps placed at various points within the system to remove condensate and air from the mobile clean steam, in downstream piping/tubing to drains, past the traps, or in condensate collectors. Reverse migration is evident in most cases, with rouge deposits forming above the traps and growing upstream into adjacent use point piping or into subheaders and beyond; the rouge that forms in traps or other components is found upstream from this source and continues to migrate both upstream and downstream.
Rouge in steam systems can be found in all forms including:
- Class 1: migrating rouge that forms in one place and migrates to another surface
- Class 2: rouge that forms on the surface where the corrosion occurs
- Class 3: rouge formed in higher-temperature conditions (over 95°C)
At use points, ball valves or valve housings exhibit significant rouge accumulation. Certain stainless steel components also demonstrate moderate to high levels of a disparate metallurgical structure, including delta ferrite. Ferrite crystal structure is suspected of lowering corrosion resistance, even though its content may only be 1%–5%. In addition, ferrite does not possess the corrosion resistance of austenitic crystal structure, therefore, it will corrode preferentially. Ferrite can be detected accurately with a ferrite meter or semi-accurately (and with significant limitations) using a magnet.
SUMMARY
From system inception, when a new CS generator and distribution tubing is first commissioned and energized, several potential factors for corrosion are present:
- In addition to clean steam, the CS generator begins to generate corrosion particles (class 1 rouge) that have the potential to migrate.
- Separately, pressure regulators begin to generate (class 3) rouge downstream, and possibly upstream as a function of time.
- High levels of delta ferrite, metallic inclusions, or other material defect content in components begin to generate corrosion products (class 2 rouge).
- Condensate traps can add further migration-capable corrosion (class 1 rouge).
- Distribution tubing will show corrosion effects and accumulated rouge (class 2 and 3 rouge).
- Ball valves can generate corrosion from trap lines as well as at use points.
Further, as a function of time, these corrosion factors may produce corrosion products as they meet, combine, and overlap with a blend of ferrous and ferric rouge. Generally, black rouge is first seen in the generator; rouge then emerges at the generator discharge piping and eventually throughout the CS distribution system.
SEM OBSERVATIONS
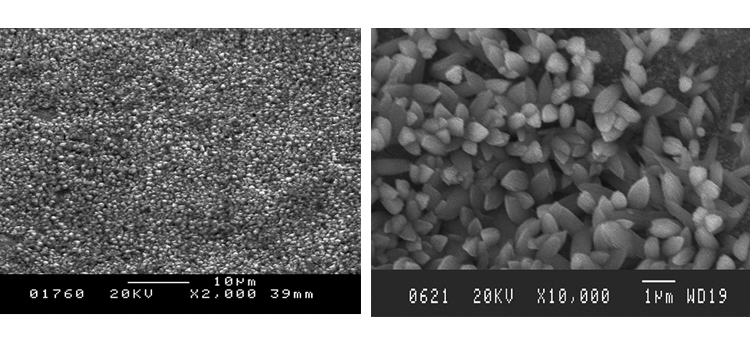
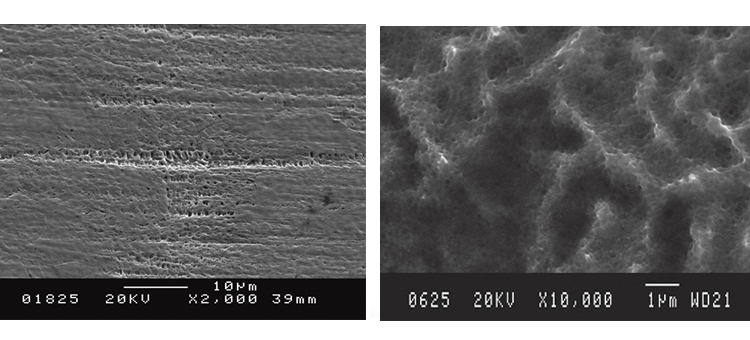
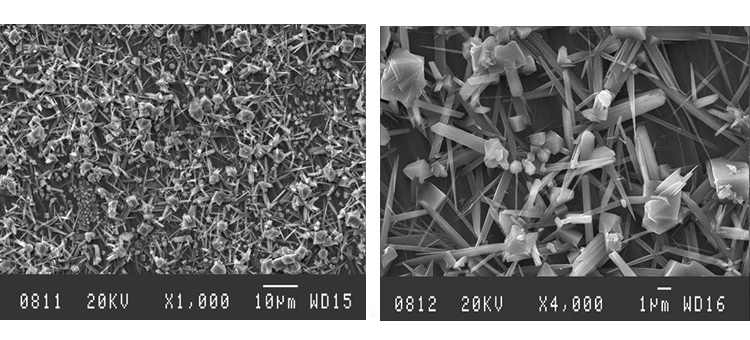
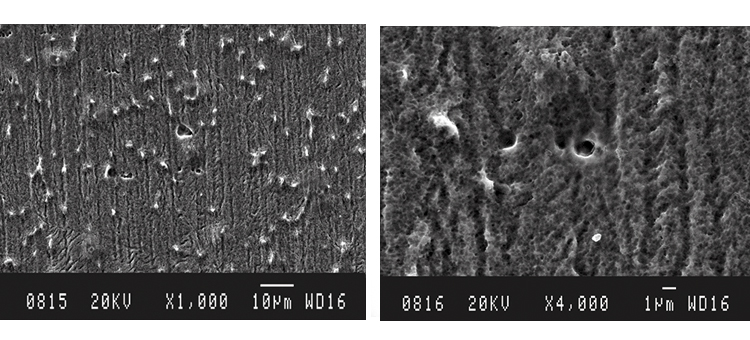
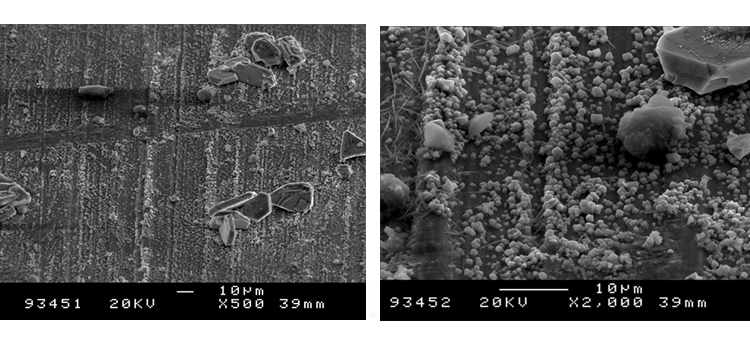
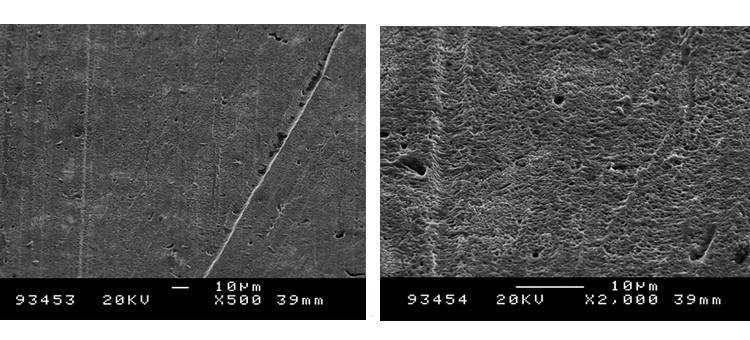
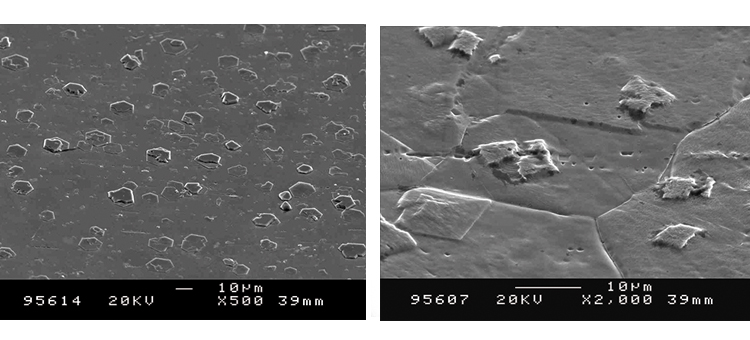
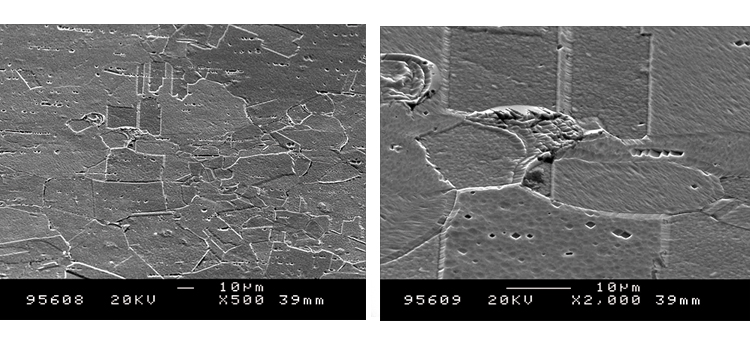
We conducted SEM analyses to illustrate the microstructure of the corrosion byproducts that covered the entire surface with crystalline and other particles. The background or underlying surface upon which the particles are distributed ranged from various gradations of ferrous (Figures 1–3) to the most ubiquitous sample, a silica/ferrous, glassy, tenacious, uniform deposit (Figure 4). A steam trap bellows (Figures 5–6), was also investigated.
AES results
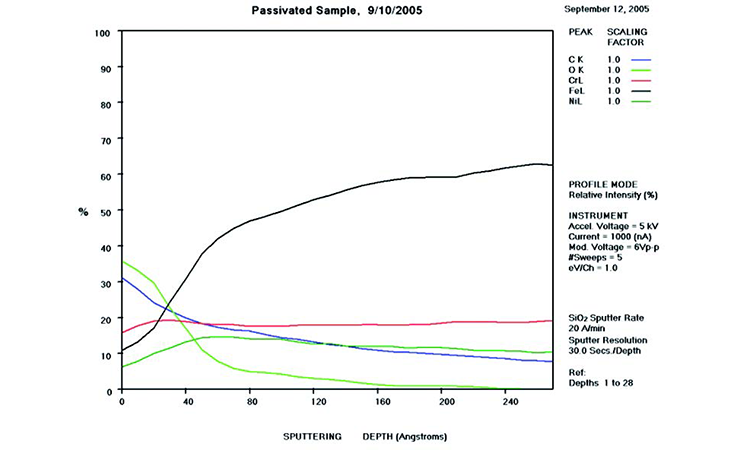
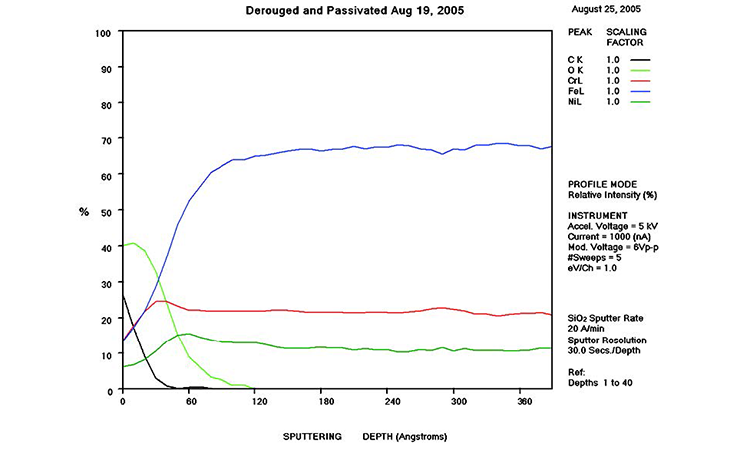
AES testing is an analytical method used to determine the surface chemistry of stainless steel and predict its corrosion resistance. It also shows the degradation of the passive film and the reduction of chromium concentration in the passive film as the surface degrades due to corrosion.
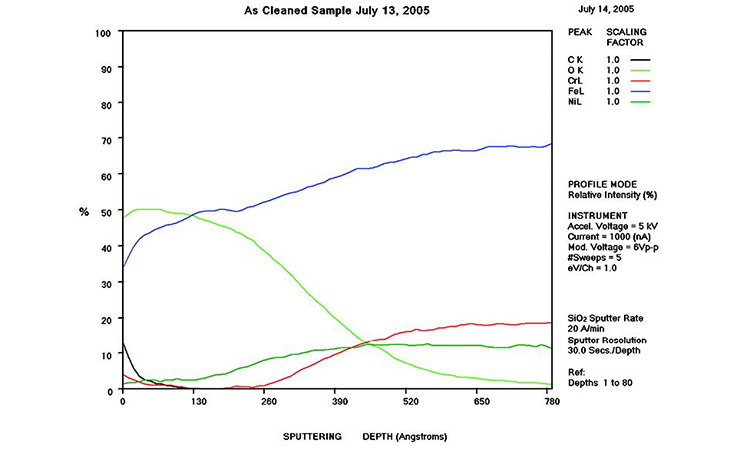
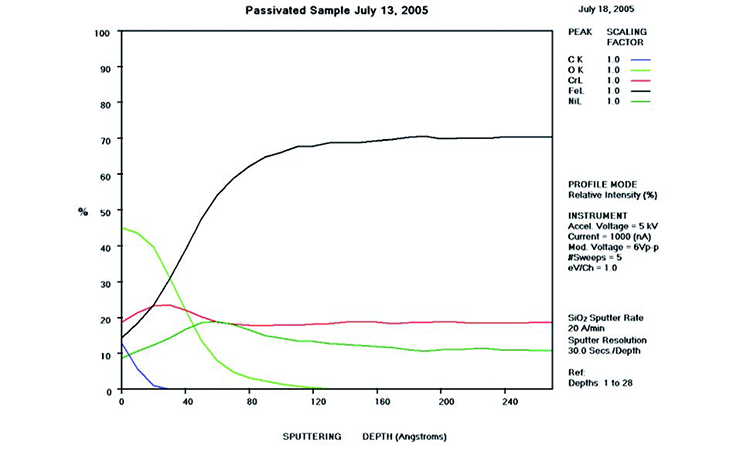
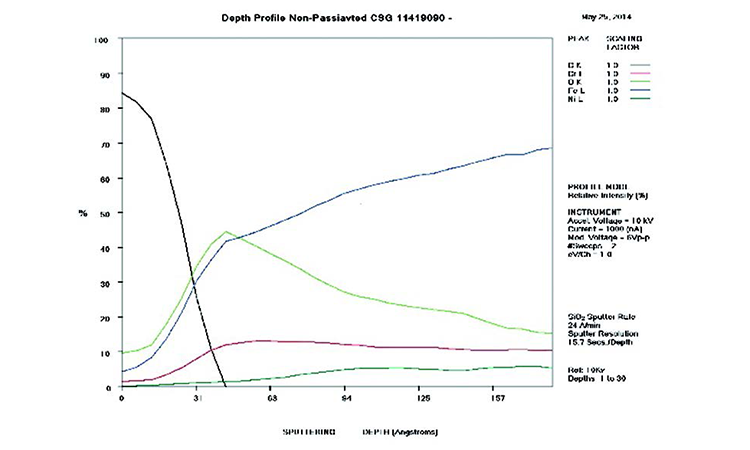
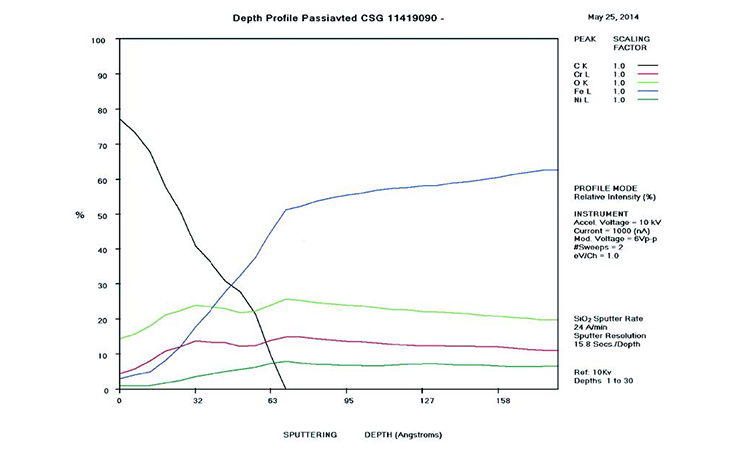
AES survey scans (depth profiles of elemental concentrations at the surface) were used to characterize the elemental composition of each sample surface. The analysis sites and SEM magnifications were carefully selected to provide information from typical regions. Each survey provided information from the top few molecular layers (estimated at 10 ångstroms [Å] per layer) down to the alloy metal depth (200–1,000 Å). Various amounts of iron (Fe), Cr (chromium), oxygen (O), nickel (Ni) and carbon (C) were found in all areas of rouge. AES figures and results are described in the Case Studies section.
Typical AES results of initial conditions show heavy oxidation on the received sample with very high Fe and O concentrations (iron oxide) and low Cr at the surface. This rouge buildup leads to particulate release and potential contamination of product and product-contact surfaces. After the rouge is removed, the “passivated” samples show complete restoration of the passive film, with Cr reaching a higher concentration than Fe, and a Cr:Fe ratio from 1.0 to 2.0 at the surface, with a distinct lack of iron oxides.